Abstract
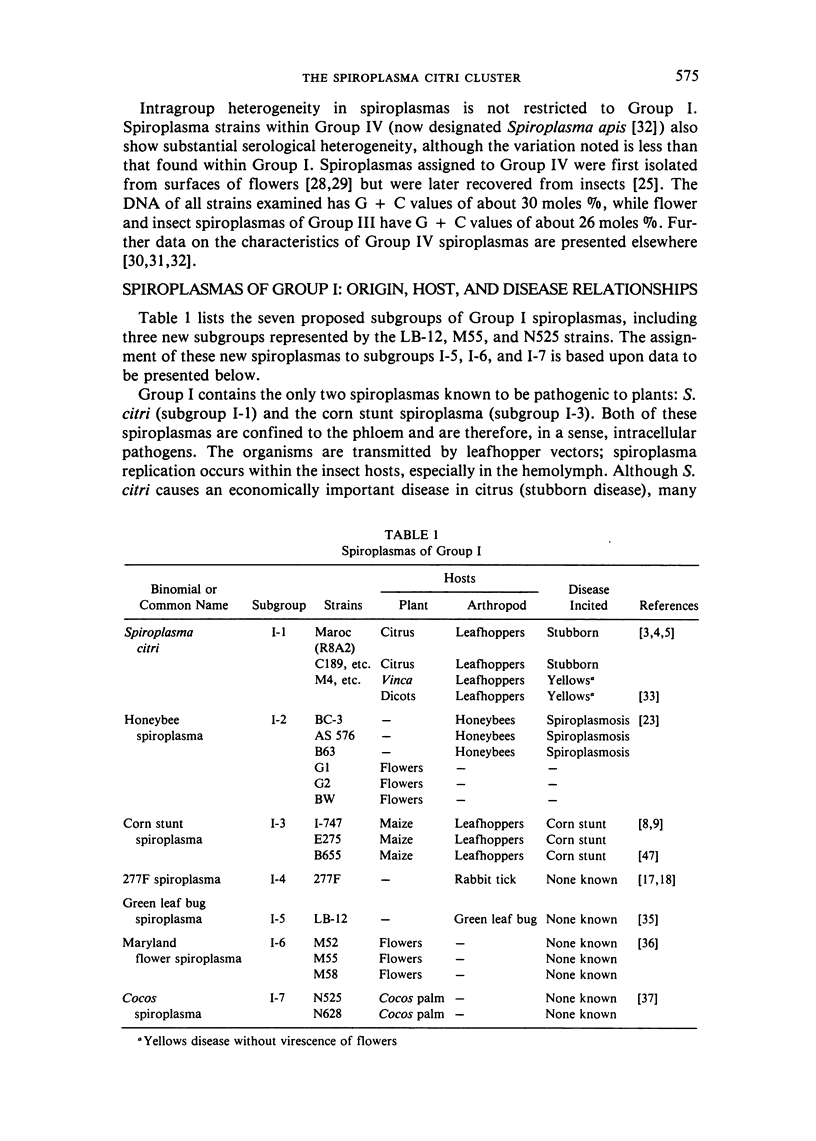
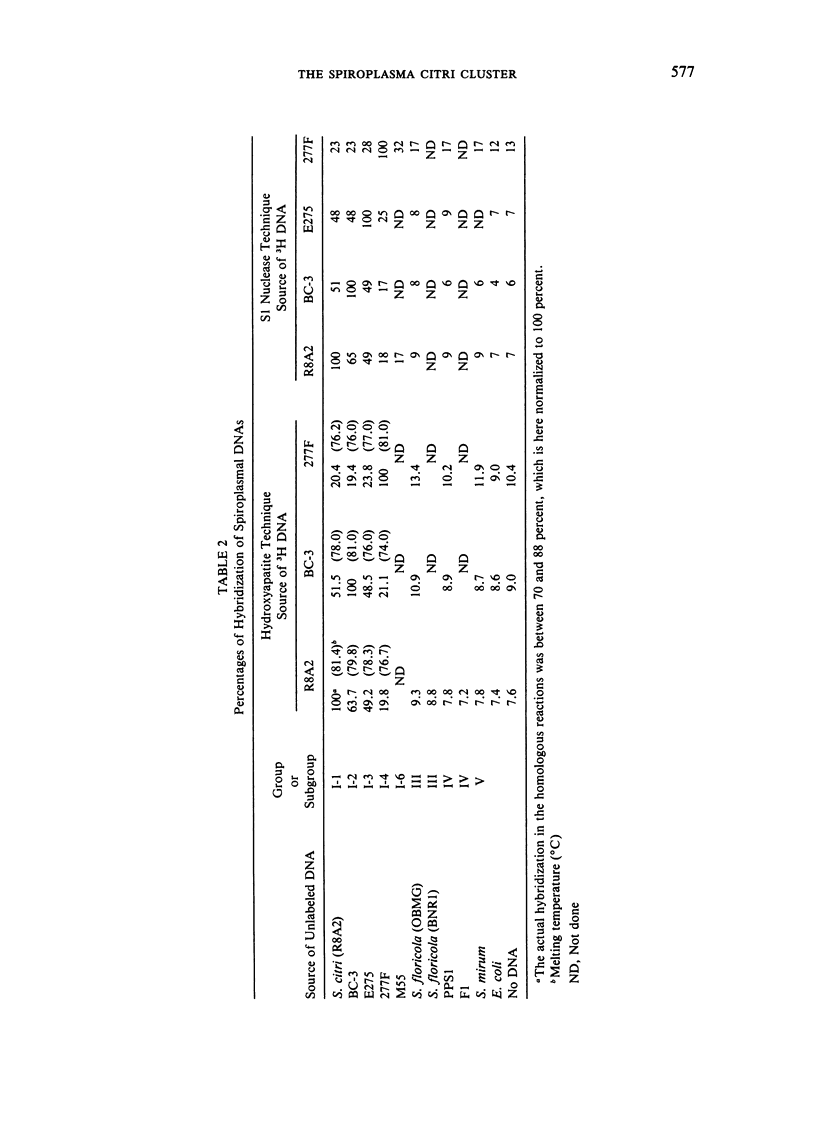
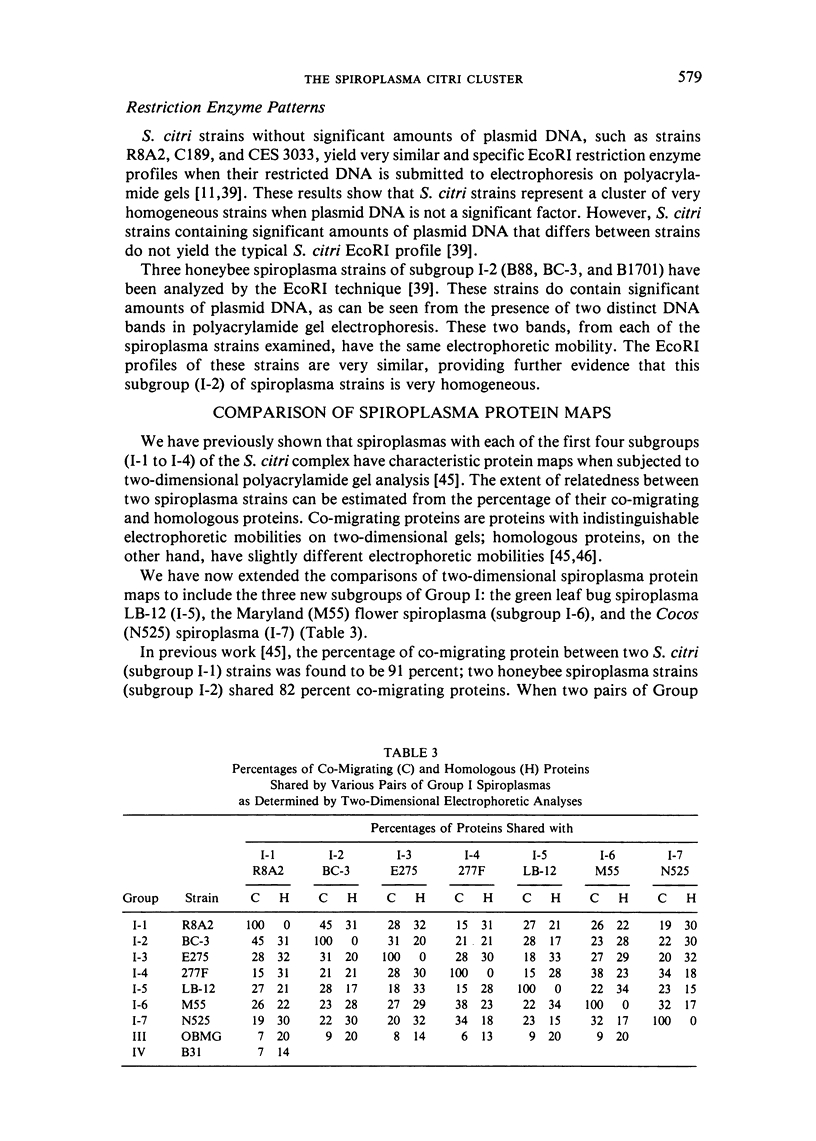
We propose that Group I spiroplasmas be subdivided into seven, rather than four, subgroups. The seven subgroups showed remarkable homogeneity when several representative strains were compared. Hybridization reactions between DNAs of representative strains within subgroups were generally at least 90 percent, and usually at least 80 percent co-migrating cell proteins were found. In addition, when plasmid DNA was excluded, profiles of restricted DNA among strains within subgroups were very similar. In contrast, comparisons between Group I subgroups showed substantial heterogeneity. This heterogeneity was indicated by DNA-DNA hybridization reactions as low as 10-20 percent and only 10-15 percent co-migrating cell proteins. Spiroplasma citri (subgroup I-1), the honeybee spiroplasma (subgroup I-2), and the corn stunt spiroplasma (subgroup I-3) are all pathogenic organisms with more or less limited host ranges. Strains of these three subgroups have been repeatedly isolated from affected hosts. Since strains of subgroups I-2 and I-3 can be clearly differentiated from other Group I subgroups and all other spiroplasmas, the DNA-DNA hybridization reactions of the subgroups do not exceed 70 percent, and because they are important pathogens, we propose (subject to completion of standard requirements for species descriptions) that they be recognized as new species of the genus Spiroplasma.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bové J. M., Saillard C., Junca P., DeGorce-Dumas J. R., Ricard B., Nhami A., Whitcomb R. F., Williamson D., Tully J. G. Guanine-plus-cytosine content, hybridization percentages, and EcoRI restriction enzyme profiles of spiroplasmal DNA. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S129–S136. doi: 10.1093/clinids/4.supplement_1.s129. [DOI] [PubMed] [Google Scholar]
- Chen T. A., Liao C. H. Corn stunt spiroplasma: isolation, cultivation, and proof of pathogenicity. Science. 1975 Jun 6;188(4192):1015–1017. doi: 10.1126/science.188.4192.1015. [DOI] [PubMed] [Google Scholar]
- Clark T. B. Spiroplasmas: diversity of arthropod reservoirs and host-parasite relationships. Science. 1982 Jul 2;217(4554):57–59. doi: 10.1126/science.217.4554.57. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Tully J. G., Popkin T. J., Bové J. M. Morphology, ultrastructure, and bacteriophage infection of the helical mycoplasma-like organism (Spiroplasma citri gen. nov., sp. nov.) cultured from "stubborn" disease of citrus. J Bacteriol. 1973 Jul;115(1):367–384. doi: 10.1128/jb.115.1.367-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. E. Spiroplasma associated with flowers of the tulip tree (Liriodendron tulipifera L.). Can J Microbiol. 1978 Aug;24(8):954–959. doi: 10.1139/m78-158. [DOI] [PubMed] [Google Scholar]
- Lee I. M., Davis R. E. DNA homology among diverse spiroplasma strains representing several serological groups. Can J Microbiol. 1980 Nov;26(11):1356–1363. doi: 10.1139/m80-224. [DOI] [PubMed] [Google Scholar]
- Mouches C., Bové J. M. A plasmid from S. citri strain M14 hybridizes with extrachromosomal DNAs from other spiroplasmas, including corn stunt spiroplasma E275, tick spiroplasma 277F, and coco spiroplasma N525. Yale J Biol Med. 1983 Sep-Dec;56(5-6):723–727. [PMC free article] [PubMed] [Google Scholar]
- Mouches C., Bové J. M., Tully J. G., Rose D. L., McCoy R. E., Carle-Junca P., Garnier M., Saillard C. Spiroplasma apis, a new species from the honey-bee Apis mellifera. Ann Microbiol (Paris) 1983 May-Jun;134A(3):383–397. [PubMed] [Google Scholar]
- Mouches C., Candresse T., McGarrity G. J., Bové J. M. Analysis of spiroplasma proteins: contribution to the taxonomy of group IV spiroplasmas and the characterization of spiroplasma protein antigens. Yale J Biol Med. 1983 Sep-Dec;56(5-6):431–437. [PMC free article] [PubMed] [Google Scholar]
- Mouches C., Menara A., Tully J. G., Bové J. M. Polyacrylamide gel analysis of spiroplasmas proteins and its contribution to the taxonomy of spiroplasmas. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S141–S147. doi: 10.1093/clinids/4.supplement_1.s141. [DOI] [PubMed] [Google Scholar]
- POULSON D. F., SAKAGUCHI B. Nature of "sex-ratio" agent in Drosophila. Science. 1961 May 12;133(3463):1489–1490. doi: 10.1126/science.133.3463.1489. [DOI] [PubMed] [Google Scholar]
- Pickens E. G., Gerloff R. K., Burgdorfer W. Spirochete from the rabbit tick, Haemaphysalis leporispalustris (Packard). I. Isolation and preliminary characterization. J Bacteriol. 1968 Feb;95(2):291–299. doi: 10.1128/jb.95.2.291-299.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju B. C., Nyland G., Meikle T., Purcell A. H. Helical, motile mycoplasmas associated with flowers and honey bees in California. Can J Microbiol. 1981 Feb;27(2):249–253. doi: 10.1139/m81-038. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Mitchell W. O., Popkin T. J., Cole R. M. Covalently closed circular deoxyribonucleic acids in spiroplasmas. J Bacteriol. 1980 Sep;143(3):1194–1199. doi: 10.1128/jb.143.3.1194-1199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Bove J. M., Saglio P. Plant Mycoplasmas: Serological Relation between Agents Associated with Citrus Stubborn and Corn Stunt Diseases. Science. 1973 Nov 23;182(4114):827–829. doi: 10.1126/science.182.4114.827. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Williamson D. L., Clark H. F. Suckling mouse cataract agent is a helical wall-free prokaryote (spiroplasma) pathogenic for vertebrates. Nature. 1976 Jan 15;259(5539):117–120. doi: 10.1038/259117a0. [DOI] [PubMed] [Google Scholar]
- Whitcomb R. F., Clark T. B., Tully J. G., Chen T. A., Bové J. M. Serological classification of spiroplasmas: current status. Yale J Biol Med. 1983 Sep-Dec;56(5-6):453–459. [PMC free article] [PubMed] [Google Scholar]
- Williamson D. L., Whitcomb R. F. Plant mycoplasmas: a cultivable spiroplasma causes corn stunt disease. Science. 1975 Jun 6;188(4192):1018–1020. doi: 10.1126/science.188.4192.1018. [DOI] [PubMed] [Google Scholar]
- Zeigel R. F., Clark H. F. Electron microscopy of the suckling mouse cataract agent: a noncultivable animal pathogen possibly related to mycoplasma. Infect Immun. 1974 Feb;9(2):430–443. doi: 10.1128/iai.9.2.430-443.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


