Abstract
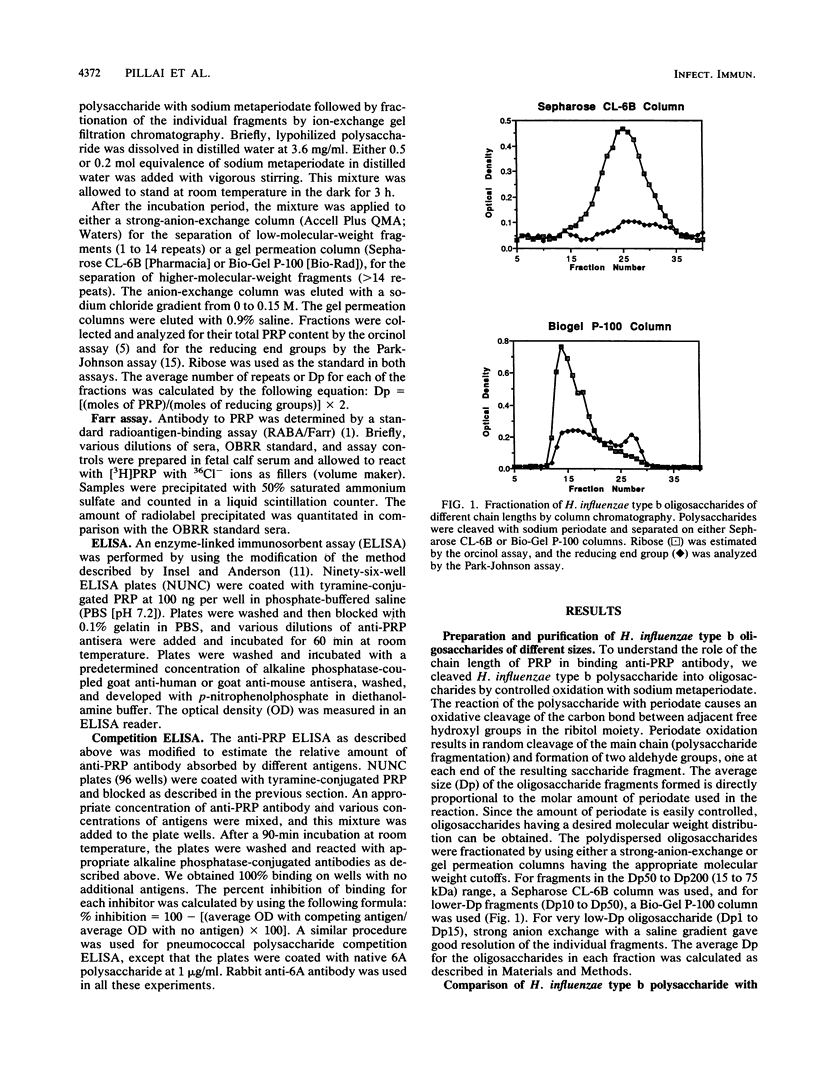
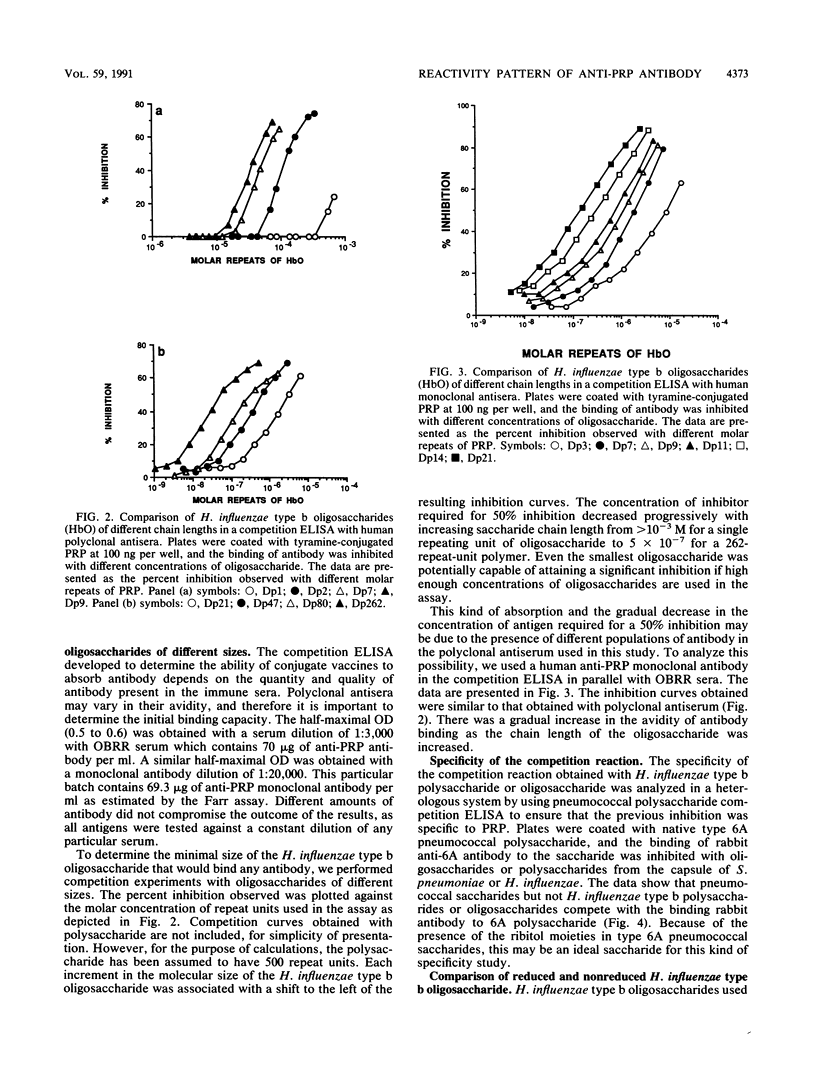
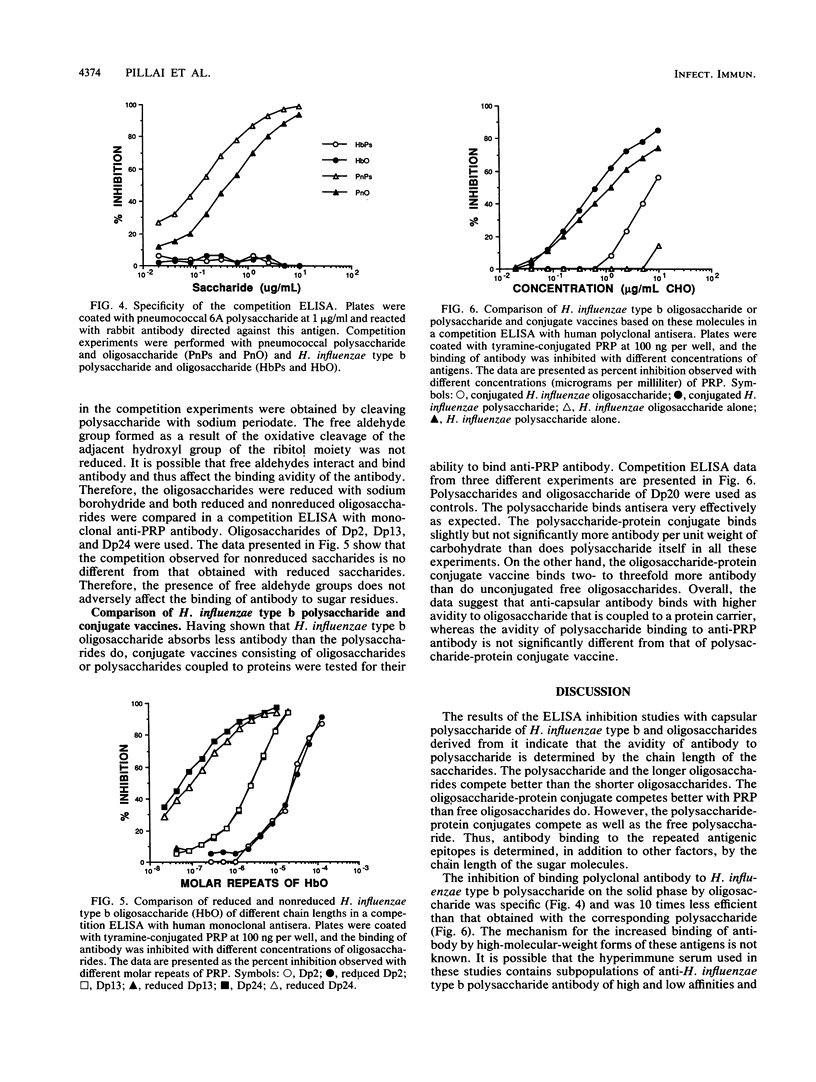
The chain length of oligosaccharides required for antibody binding has been studied by using the capsular polysaccharide from Haemophilus influenzae type b or oligosaccharides derived from it. The concentration of competing antigens required to achieve a 50% inhibition of antibody binding by human polyclonal antisera in an in vitro competition enzyme-linked immunosorbent assay decreased progressively from greater than 10(-3) to 5 x 10(-7) M as the inhibiting saccharide chain length increased from 1 to 262 repeat units. Even small oligosaccharides (one or two repeat units) are potentially capable of competing to a significant level if a high enough concentration of saccharides is used. A similar pattern of reactivity was seen with a monoclonal anti-polyribosyl ribitol phosphate antibody, suggesting that the differences in the avidity of the antibody subpopulations in the polyclonal antisera do not contribute to the binding patterns observed. The binding reaction was specific as evaluated with pneumococcal saccharides. Furthermore, an oligosaccharide-protein conjugate binds antibody better than the free oligosaccharides do. Such a difference in binding was not observed between the polysaccharide and a polysaccharide-protein conjugate. Overall, the data suggest that identical epitopes are expressed by oligomeric and polymeric forms of the antigen and that a particularly more stable conformation in polysaccharides is preferred by antibodies. Covalent coupling of oligomers to protein increases the expression of stable conformation of epitopes. The data further suggest that this kind of antigenic analysis may be important for the design and synthesis of glycoconjugate vaccines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Stein E. C., Porcelli S., Betts R. F., Connuck D. M., Korones D., Insel R. A., Zahradnik J. M., Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J Immunol. 1989 Apr 1;142(7):2464–2468. [PubMed] [Google Scholar]
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. Intrinsic tritium labeling of the capsular polysaccharide antigen of Haemophilus influenzae type B. J Immunol. 1978 Mar;120(3):866–870. [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structures of proteins. Their determination has revealed important aspects of immune recognition and generated strategies for synthetic mimicking of protein binding sites. Eur J Biochem. 1984 Nov 15;145(1):1–20. doi: 10.1111/j.1432-1033.1984.tb08516.x. [DOI] [PubMed] [Google Scholar]
- Glaudemans C. P. Seven structurally different murine monoclonal galactan-specific antibodies show identity in their galactosyl-binding subsite arrangements. Mol Immunol. 1987 Apr;24(4):371–377. doi: 10.1016/0161-5890(87)90179-9. [DOI] [PubMed] [Google Scholar]
- Gordon L. K. Studies on the combined administration of Haemophilus influenzae type B--diphtheria toxoid conjugate vaccine (PRP-D) and DTP. Dev Biol Stand. 1986;65:113–121. [PubMed] [Google Scholar]
- Greenspan N. S., Monafo W. J., Davie J. M. Interaction of IgG3 anti-streptococcal group A carbohydrate (GAC) antibody with streptococcal group A vaccine: enhancing and inhibiting effects of anti-GAC, anti-isotypic, and anti-idiotypic antibodies. J Immunol. 1987 Jan 1;138(1):285–292. [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985 Apr;134(4):2651–2657. [PubMed] [Google Scholar]
- Kabat E. A., Liao J., Osserman E. F., Gamian A., Michon F., Jennings H. J. The epitope associated with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia coli K1 to a human monoclonal macroglobulin, IgMNOV. J Exp Med. 1988 Aug 1;168(2):699–711. doi: 10.1084/jem.168.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Seppälä I., Pelkonen J., Mäkelä O. Isotypes of antibodies induced by plain dextran or a dextran-protein conjugate. Eur J Immunol. 1985 Aug;15(8):827–833. doi: 10.1002/eji.1830150816. [DOI] [PubMed] [Google Scholar]
- Sharon J., Kabat E. A., Morrison S. L. Immunochemical characterization of binding sites of hybridoma antibodies specific for alpha (1 leads to 6) linked dextran. Mol Immunol. 1982 Mar;19(3):375–388. doi: 10.1016/0161-5890(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Wessels M. R., Kasper D. L. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J Exp Med. 1989 Jun 1;169(6):2121–2131. doi: 10.1084/jem.169.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Muñoz A., Kasper D. L. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9170–9174. doi: 10.1073/pnas.84.24.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter W. T., Smith P. J., Arnott S. Hyaluronic acid: structure of a fully extended 3-fold helical sodium salt and comparison with the less extended 4-fold helical forms. J Mol Biol. 1975 Dec 5;99(2):219–235. doi: 10.1016/s0022-2836(75)80142-2. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Bacon B. Three-dimensional structural analysis of the group B polysaccharide of Neisseria meningitidis 6275 by two-dimensional NMR: the polysaccharide is suggested to exist in helical conformations in solution. Biochemistry. 1991 Jan 22;30(3):851–857. doi: 10.1021/bi00217a039. [DOI] [PubMed] [Google Scholar]


