Abstract
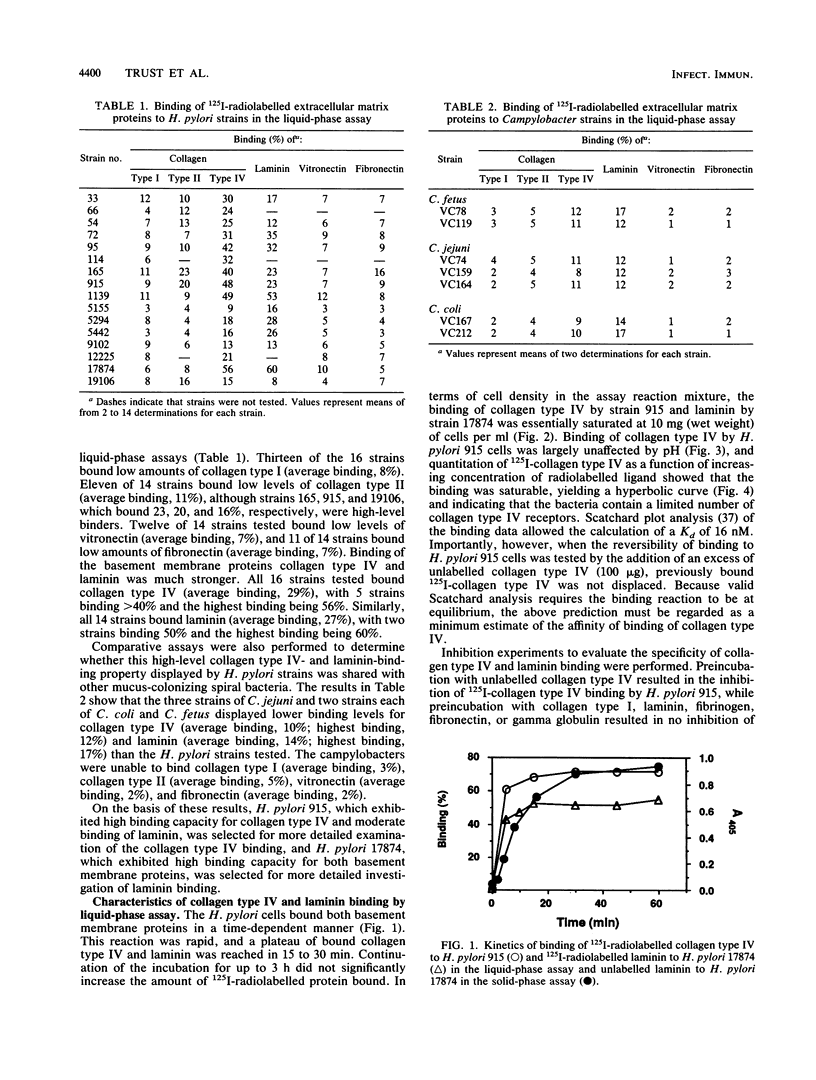
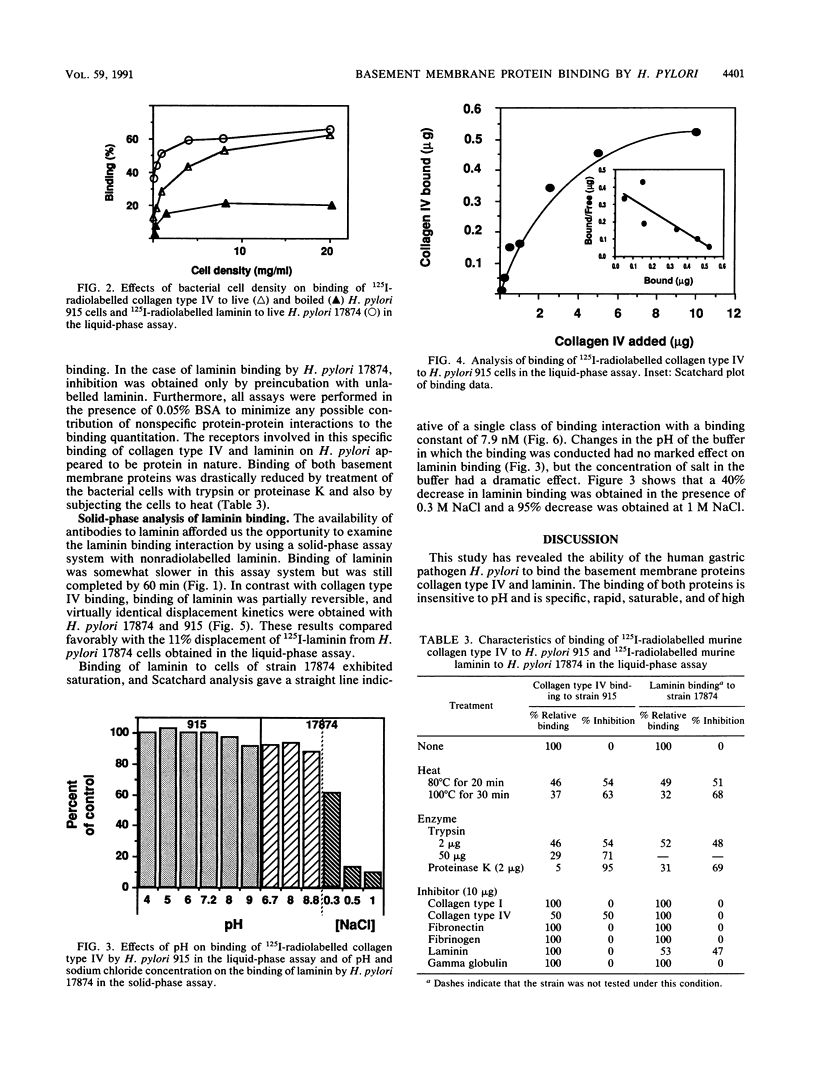
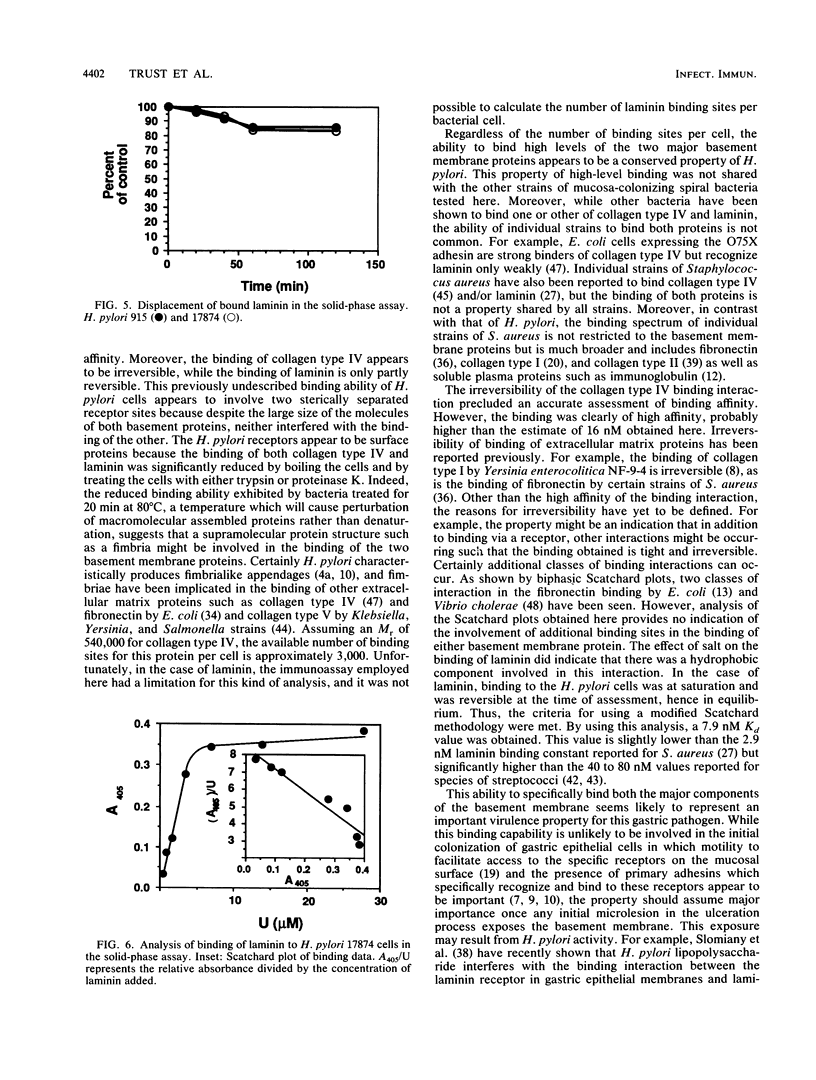
The ability of 16 isolates of the human gastroduodenal pathogen Helicobacter pylori to bind 125I-radiolabelled tissue proteins was quantitated by liquid-phase assay. While capable of binding generally low levels of collagen types I and II, vitronectin, and fibronectin (average binding, 8%; highest binding, 23%), the various H. pylori isolates were good binders of the basement membrane proteins collagen type IV and laminin (average binding, 27%; highest binding, 60%). Campylobacter species tested bound lower levels of collagen type IV and laminin (average binding, 12%; highest binding, 17%). Trypsin and proteinase K treatment of H. pylori cells markedly reduced the binding of collagen type IV and laminin, as did heat treatment, suggesting that the binding of basement membrane proteins is mediated by bacterial surface proteins. Binding of both basement membrane proteins was rapid and saturable. 125I-collagen type IV binding to H. pylori 915 was inhibited by preincubation with unlabelled collagen type IV but was not inhibited by laminin or a number of other proteins. Once bound, radiolabelled collagen type IV but was not displaced by an excess of unlabelled collagen type IV, indicating that the binding interaction was of high affinity. Binding of laminin was partially reversible, and analysis in a solid-phase nonradiolabel assay showed that the interaction was of high affinity, with a Kd of 7.9 nM. This interaction was affected by salt, indicating the presence of a hydrophobic component in the ability of H. pylori to bind laminin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987 Aug;93(2):371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- Buck G. E., Gourley W. K., Lee W. K., Subramanyam K., Latimer J. M., DiNuzzo A. R. Relation of Campylobacter pyloridis to gastritis and peptic ulcer. J Infect Dis. 1986 Apr;153(4):664–669. doi: 10.1093/infdis/153.4.664. [DOI] [PubMed] [Google Scholar]
- Coghlan J. G., Gilligan D., Humphries H., McKenna D., Dooley C., Sweeney E., Keane C., O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987 Nov 14;2(8568):1109–1111. doi: 10.1016/s0140-6736(87)91545-5. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Podack E. R. Characterization of human S protein, an inhibitor of the membrane attack complex of complement. Demonstration of a free reactive thiol group. Biochemistry. 1985 Apr 23;24(9):2368–2374. doi: 10.1021/bi00330a036. [DOI] [PubMed] [Google Scholar]
- Dooley C. P., Cohen H. The clinical significance of Campylobacter pylori. Ann Intern Med. 1988 Jan;108(1):70–79. doi: 10.7326/0003-4819-108-1-70. [DOI] [PubMed] [Google Scholar]
- Dubreuil J. D., Logan S. M., Cubbage S., Eidhin D. N., McCubbin W. D., Kay C. M., Beveridge T. J., Ferris F. G., Trust T. J. Structural and biochemical analyses of a surface array protein of Campylobacter fetus. J Bacteriol. 1988 Sep;170(9):4165–4173. doi: 10.1128/jb.170.9.4165-4173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emödy L., Carlsson A., Ljungh A., Wadström T. Mannose-resistant haemagglutination by Campylobacter pylori. Scand J Infect Dis. 1988;20(3):353–354. doi: 10.3109/00365548809032466. [DOI] [PubMed] [Google Scholar]
- Emödy L., Heesemann J., Wolf-Watz H., Skurnik M., Kapperud G., O'Toole P., Wadström T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989 Dec;171(12):6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayers. Infect Immun. 1989 Aug;57(8):2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Armstrong J. A., Marshall B. J. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986 Apr;39(4):353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Klein P. D. Campylobacter pyloridis gastritis: the past, the present, and speculations about the future. Am J Gastroenterol. 1987 Apr;82(4):283–286. [PubMed] [Google Scholar]
- Hamilton I., O'Connor H. J., Wood N. C., Bradbury I., Axon A. T. Healing and recurrence of duodenal ulcer after treatment with tripotassium dicitrato bismuthate (TDB) tablets or cimetidine. Gut. 1986 Jan;27(1):106–110. doi: 10.1136/gut.27.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handfield-Jones S. E., Kennedy C. T., Bradfield J. B. Angiosarcoma arising in an angiomatous naevus following irradiation in childhood. Br J Dermatol. 1988 Jan;118(1):109–112. doi: 10.1111/j.1365-2133.1988.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Harris L. A., Logan S. M., Guerry P., Trust T. J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987 Nov;169(11):5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Holderbaum D., Spech R. A., Ehrhart L. A. Specific binding of collagen to Staphylococcus aureus. Coll Relat Res. 1985 Jun;5(3):261–271. doi: 10.1016/s0174-173x(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Jiang S. J., Liu W. Z., Zhang D. Z., Shi Y., Xiao S. D., Zhang Z. H., Lu D. Y. Campylobacter-like organisms in chronic gastritis, peptic ulcer, and gastric carcinoma. Scand J Gastroenterol. 1987 Jun;22(5):553–558. doi: 10.3109/00365528708991897. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pyloridis and gastritis. J Infect Dis. 1986 Apr;153(4):650–657. doi: 10.1093/infdis/153.4.650. [DOI] [PubMed] [Google Scholar]
- McNulty C. A. Campylobacter pyloridis-associated gastritis. J Infect. 1986 Sep;13(2):107–113. doi: 10.1016/s0163-4453(86)92757-x. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Faragher E. B. Relapse of duodenal ulcer: does it matter which drug is used in initial treatment? Br Med J (Clin Res Ed) 1986 Nov 1;293(6555):1117–1118. doi: 10.1136/bmj.293.6555.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Ishibashi M., Konishi H., Takemoto T., Shigeeda M., Kochiyama T. Hemagglutination activity of Campylobacter pylori. Infect Immun. 1989 Mar;57(3):989–991. doi: 10.1128/iai.57.3.989-991.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989 Apr 20;338(6217):652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- Price A. B., Levi J., Dolby J. M., Dunscombe P. L., Smith A., Clark J., Stephenson M. L. Campylobacter pyloridis in peptic ulcer disease: microbiology, pathology, and scanning electron microscopy. Gut. 1985 Nov;26(11):1183–1188. doi: 10.1136/gut.26.11.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Slomiany B. L., Piotrowski J., Sengupta S., Slomiany A. Inhibition of gastric mucosal laminin receptor by Helicobacter pylori lipopolysaccharide. Biochem Biophys Res Commun. 1991 Mar 29;175(3):963–970. doi: 10.1016/0006-291x(91)91659-z. [DOI] [PubMed] [Google Scholar]
- Speziale P., Raucci G., Visai L., Switalski L. M., Timpl R., Hök M. Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986 Jul;167(1):77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer H. W. Surface morphology of the gastroduodenal mucosa in duodenal ulceration. Gut. 1984 Nov;25(11):1203–1210. doi: 10.1136/gut.25.11.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Tarkkanen A. M., Allen B. L., Westerlund B., Holthöfer H., Kuusela P., Risteli L., Clegg S., Korhonen T. K. Type V collagen as the target for type-3 fimbriae, enterobacterial adherence organelles. Mol Microbiol. 1990 Aug;4(8):1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., Kuusela P., Risteli J., Risteli L., Vartio T., Rauvala H., Virkola R., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989 Mar;3(3):329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]


