Abstract
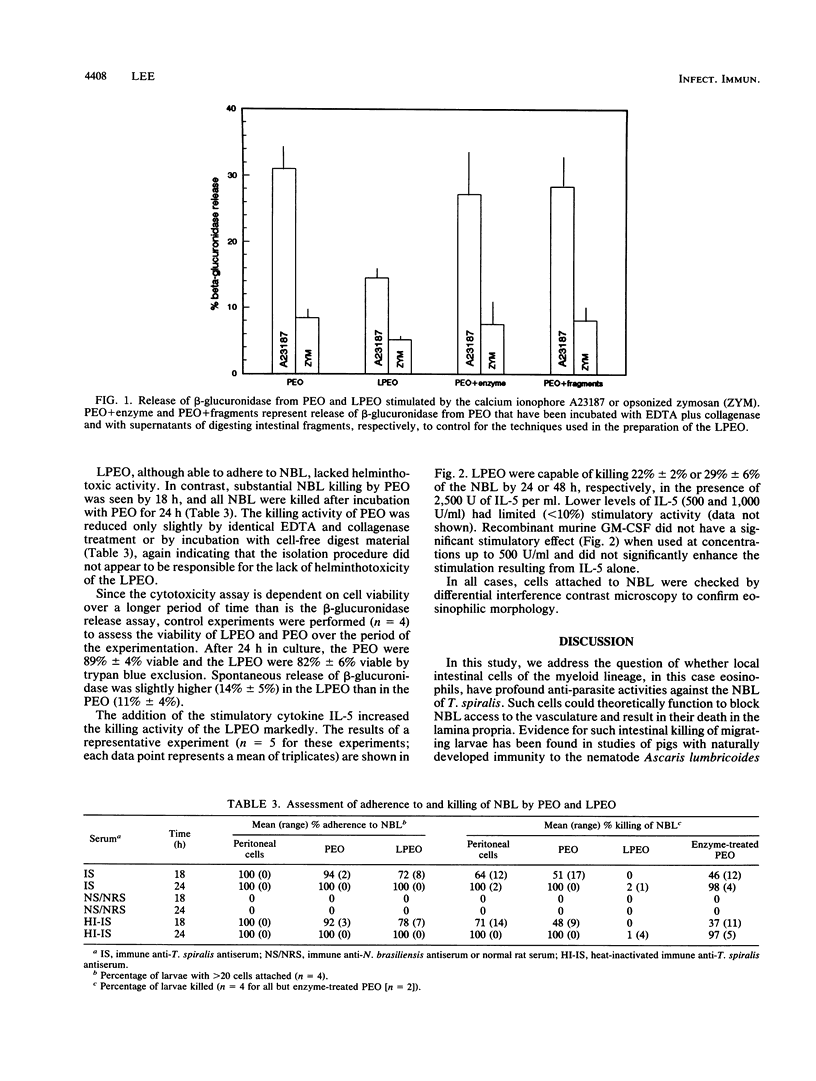
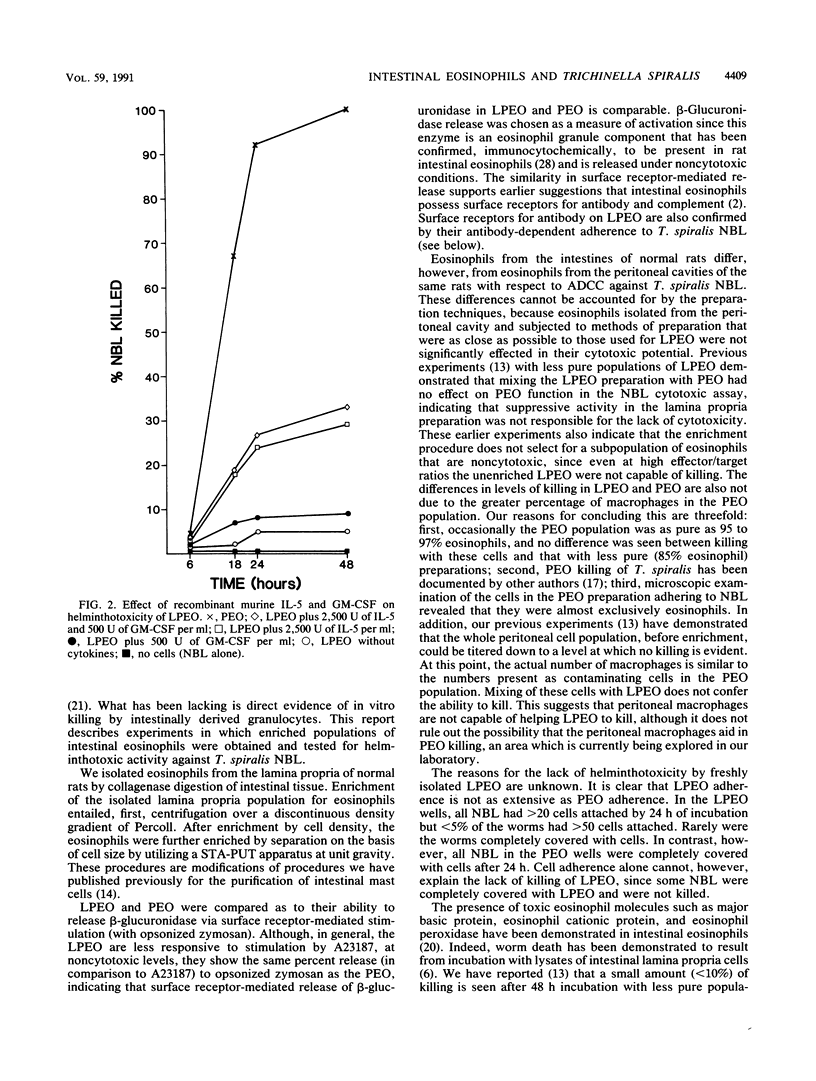
Because the gastrointestinal lamina propria is the first line of defense against invasion with Trichinella spiralis newborn larvae, we investigated the helminthotoxic characteristics of isolated lamina propria eosinophils. Eosinophils were isolated from the intestinal lamina propria of rats and purified to nearly 90% purity by a combination of velocity sedimentation through Percoll and unit gravity sedimentation through a continuous gradient of bovine serum albumin. Isolated eosinophils were of high viability and responded to surface receptor stimulation. Freshly isolated intestinal eosinophils lacked cytotoxic capacity when incubated with newborn larvae in the presence of specific antiserum. Peritoneal eosinophils from the same rats exhibited 100% helminthotoxicity after 24 h. Cytotoxicity could be stimulated in the intestinal eosinophils by the addition of recombinant murine interleukin-5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Szejda P. Mechanisms of killing of newborn larvae of Trichinella spiralis by neutrophils and eosinophils. Killing by generators of hydrogen peroxide in vitro. J Clin Invest. 1979 Dec;64(6):1558–1564. doi: 10.1172/JCI109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeken W. L., Northwood I., Beliveau C., Baigent G., Gump D. Eosinophils of human colonic mucosa: C3b and Fc gamma receptor expression and phagocytic capabilities. Clin Immunol Immunopathol. 1987 Jun;43(3):289–300. doi: 10.1016/0090-1229(87)90138-3. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Dyck N., Goodacre R., Bienenstock J. Mast cells from the human intestinal lamina propria. Isolation, histochemical subtypes, and functional characterization. J Immunol. 1987 Apr 15;138(8):2604–2610. [PubMed] [Google Scholar]
- Befus A. D., Pearce F. L., Gauldie J., Horsewood P., Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982 Jun;128(6):2475–2480. [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- Castro G. A., Roy S. A., Schanbacher L. M. Lethality of disrupted intestinal lamina propia cells for Trichinella spiralis in vitro. J Parasitol. 1975 Dec;61(6):1053–1060. [PubMed] [Google Scholar]
- Gansmuller A., Anteunis A., Venturiello S. M., Bruschi F., Binaghi R. A. Antibody-dependent in-vitro cytotoxicity of newborn Trichinella spiralis larvae: nature of the cells involved. Parasite Immunol. 1987 May;9(3):281–292. doi: 10.1111/j.1365-3024.1987.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Gardiner C. H. Habitat and reproductive behavior of Trichinella spiralis. J Parasitol. 1976 Dec;62(6):865–870. [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Kazura J. W. Host defense mechanisms against nematode parasites: destruction of newborn Trichinella spiralis larvae by human antibodies and granulocytes. J Infect Dis. 1981 May;143(5):712–718. doi: 10.1093/infdis/143.5.712. [DOI] [PubMed] [Google Scholar]
- Lee T. D., Befus D. Effects of rat and human intestinal lamina propria cells on viability and muscle establishment of Trichinella spiralis newborn larvae. J Parasitol. 1989 Feb;75(1):124–128. [PubMed] [Google Scholar]
- Lee T. D., Shanahan F., Miller H. R., Bienenstock J., Befus A. D. Intestinal mucosal mast cells: isolation from rat lamina propria and purification using unit gravity velocity sedimentation. Immunology. 1985 Aug;55(4):721–728. [PMC free article] [PubMed] [Google Scholar]
- Lee T. D., Swieter M., Bienenstock J., Befus A. D. Heterogeneity in mast cell populations. Clin Immunol Rev. 1985;4(2):143–199. [PubMed] [Google Scholar]
- Mackenzie C. D., Jungery M., Taylor P. M., Ogilvie B. M. Activation of complement, the induction of antibodies to the surface of nematodes and the effect of these factors and cells on worm survival in vitro. Eur J Immunol. 1980 Aug;10(8):594–601. doi: 10.1002/eji.1830100805. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W., Johnson G. R., Nicola N. A., Nice E. C., DeLamarter J., Thatcher D. R., Mermod J. J. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF. J Cell Physiol. 1986 Sep;128(3):421–431. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- Silberstein D. S., Owen W. F., Gasson J. C., DiPersio J. F., Golde D. W., Bina J. C., Soberman R., Austen K. F., David J. R. Enhancement of human eosinophil cytotoxicity and leukotriene synthesis by biosynthetic (recombinant) granulocyte-macrophage colony-stimulating factor. J Immunol. 1986 Nov 15;137(10):3290–3294. [PubMed] [Google Scholar]
- Torpier G., Colombel J. F., Mathieu-Chandelier C., Capron M., Dessaint J. P., Cortot A., Paris J. C., Capron A. Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clin Exp Immunol. 1988 Dec;74(3):404–408. [PMC free article] [PubMed] [Google Scholar]
- Urban J. F., Jr The epidemiology and control of swine parasites. Immunity and vaccines. Vet Clin North Am Food Anim Pract. 1986 Nov;2(3):765–778. doi: 10.1016/s0749-0720(15)31218-4. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Wang C. H., Bell R. G. Trichinella spiralis: newborn larval migration route in rats reexamined. Exp Parasitol. 1986 Feb;61(1):76–85. doi: 10.1016/0014-4894(86)90137-2. [DOI] [PubMed] [Google Scholar]
- Wang C. H., Bell R. G. Trichinella spiralis: vascular recirculation and organ retention of newborn larvae in rats. Exp Parasitol. 1986 Dec;62(3):430–441. doi: 10.1016/0014-4894(86)90052-4. [DOI] [PubMed] [Google Scholar]
- Wassom D. L., Gleich G. J. Damage to Trichinella spiralis newborn larvae by eosinophil major basic protein. Am J Trop Med Hyg. 1979 Sep;28(5):860–863. [PubMed] [Google Scholar]
- Wright K. A. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979 Jun;65(3):441–445. [PubMed] [Google Scholar]
- Yokota S., Tsuji H., Kato K. Localization of lysosomal and peroxisomal enzymes in the specific granules of rat intestinal eosinophil leukocytes revealed by immunoelectron microscopic techniques. J Histochem Cytochem. 1984 Mar;32(3):267–274. doi: 10.1177/32.3.6693757. [DOI] [PubMed] [Google Scholar]


