Abstract
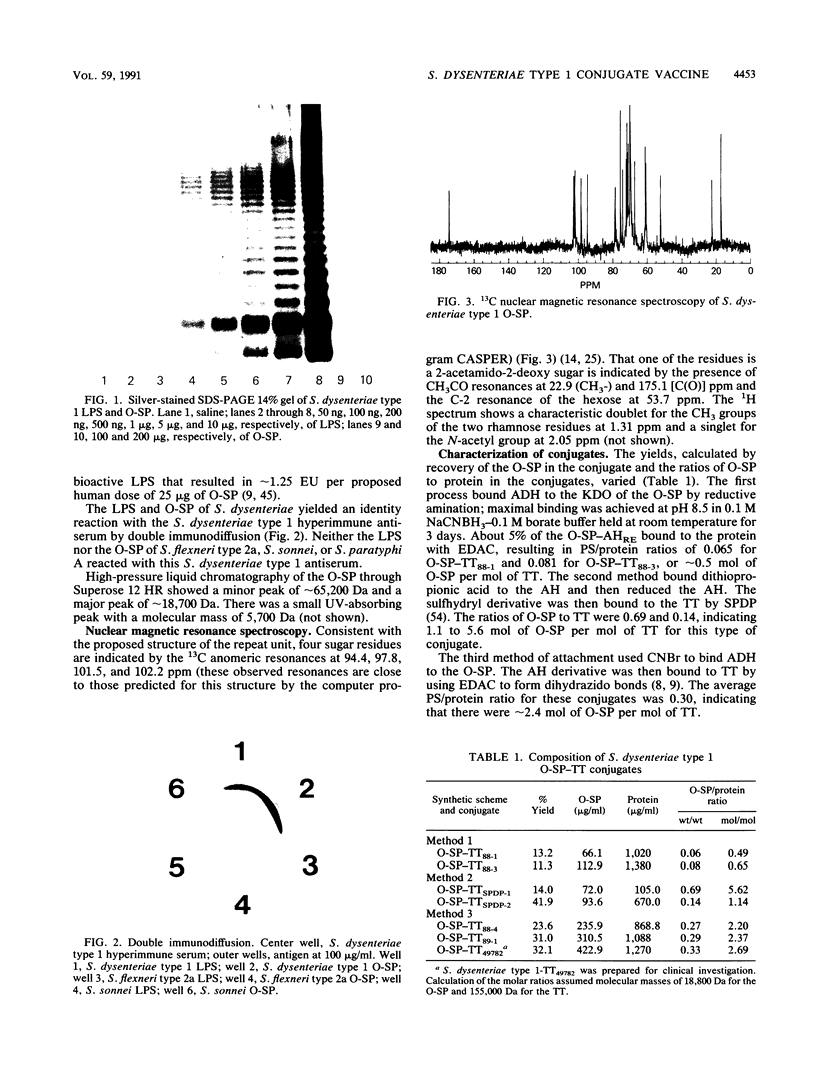
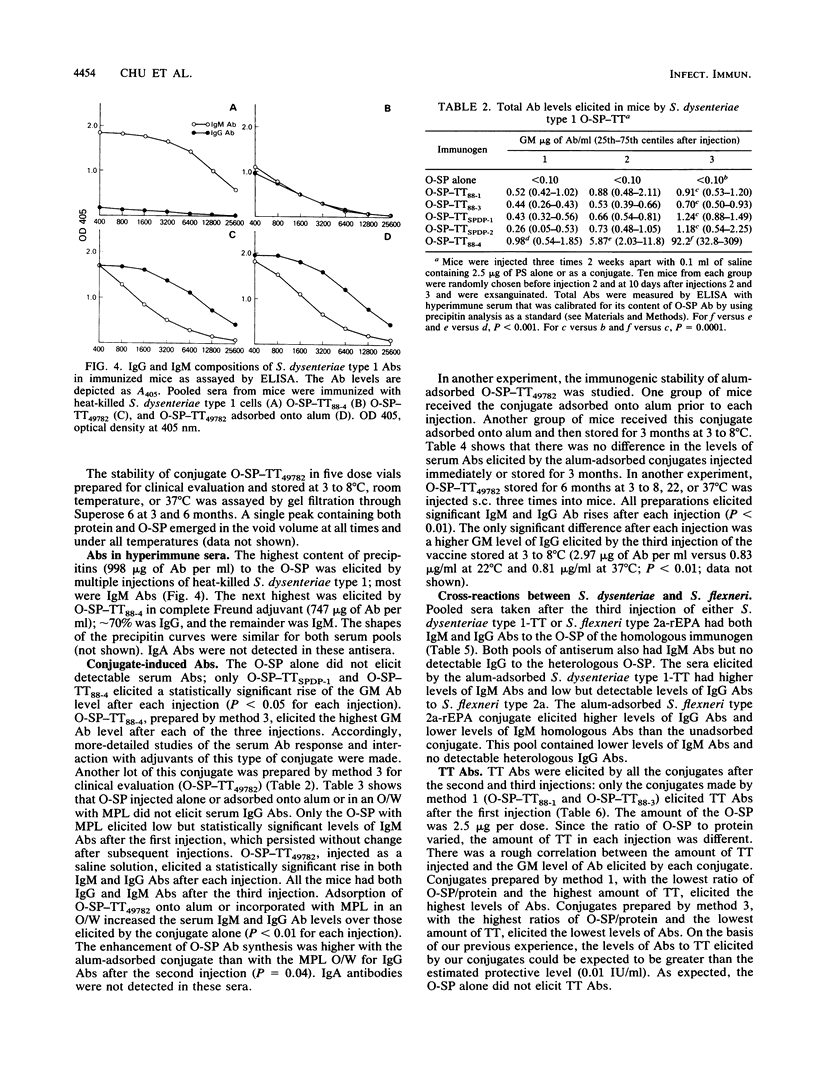
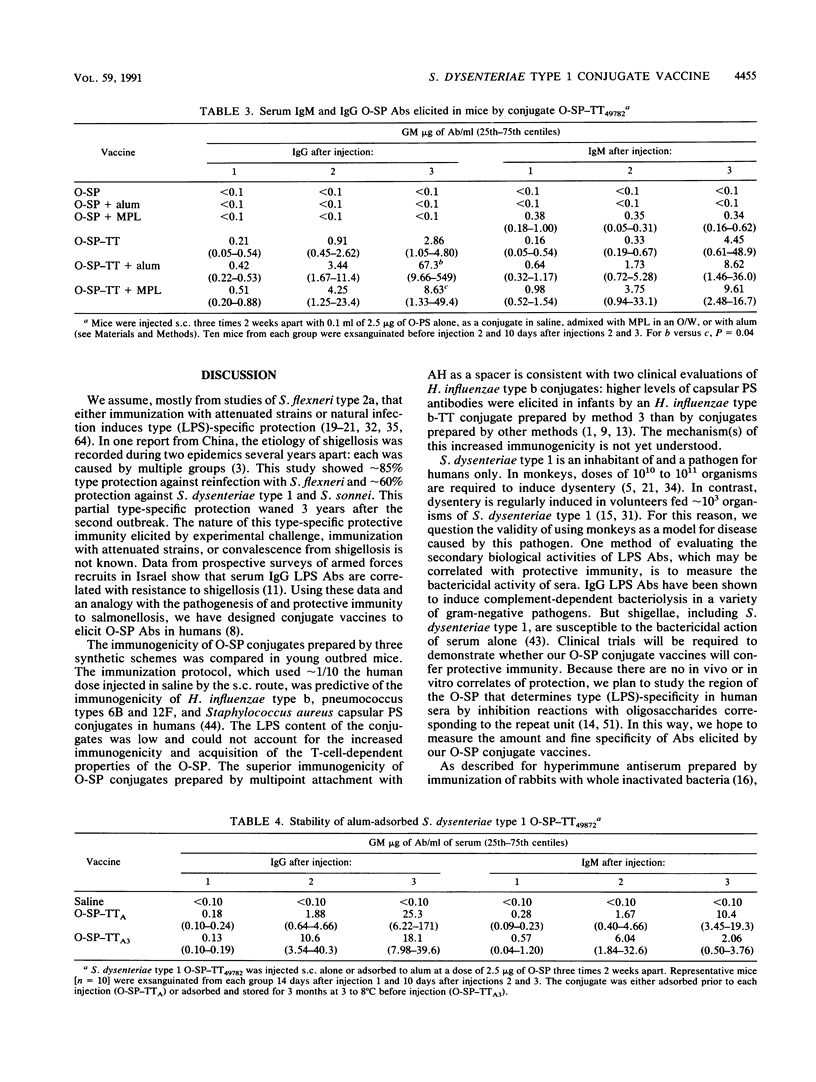
The background for developing conjugate vaccines for shigellosis composed of the O-specific polysaccharide (O-SP) bound to a protein is described elsewhere (C. Y. Chu, R. Schneerson, and J. B. Robbins, submitted for publication). Briefly, there is direct evidence for type (lipopolysaccharide [LPS])-specific protection after infection with the wild type or with attenuated strains of shigellae. Prospective studies of Israeli armed forces recruits show a correlation between preexisting serum immunoglobulin G (IgG) LPS antibodies and resistance to shigellosis (D. Cohen, M. S. Green, C. Block, R. Slephon, and I. Ofek, J. Clin. Microbiol. 29:386-389, 1991). In order to elicit IgG LPS-specific antibodies to Shigella dysenteriae type 1, the O-SP of this pathogen was purified and bound to tetanus toxoid (TT) by three schemes. The most immunogenic used a modification of a published method (C. Y. Chu, R. Schneerson, J. B. Robbins, and S. C. Rastogi, Infect. Immun. 40:245-256, 1983). The resultant O-SP-TT conjugates were stable and elicited high levels of IgG O-SP antibodies and booster responses in young mice when injected subcutaneously in saline at 1/10 the proposed human dose. Adsorption onto alum or concurrent administration with monophosphoryl lipid A enhanced both the IgG and IgM antibody responses to the O-SP of the conjugate; both the nonadsorbed and adsorbed conjugates elicited higher rises of IgG than of IgM antibodies. Clinical evaluations of S. dysenteriae type 1 O-SP-TT conjugates are planned.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. W., Pichichero M. E., Stein E. C., Porcelli S., Betts R. F., Connuck D. M., Korones D., Insel R. A., Zahradnik J. M., Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J Immunol. 1989 Apr 1;142(7):2464–2468. [PubMed] [Google Scholar]
- BRANHAM S. E., HABEL K., LILLIE R. D. Studies with Shigella dysenteriae (Shiga) infection and intoxication in Macacus mulatta monkeys. J Infect Dis. 1949 Nov-Dec;85(3):295–303. doi: 10.1093/infdis/85.3.295. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. K., Sen D., Nair G. B., Bhattacharya M. K., Datta P., Datta D. Multiply drug-resistant Shigella dysenteriae type 1 and travelers' diarrhea. J Infect Dis. 1986 Oct;154(4):729–730. doi: 10.1093/infdis/154.4.729. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Vaughan S., Timmis K. N. 'O'-antigens are essential virulence factors of Shigella sonnei and Shigella dysenteriae 1. Zentralbl Bakteriol Mikrobiol Hyg B. 1985 Jun;181(1-2):197–205. [PubMed] [Google Scholar]
- Carlin N. I., Lindberg A. A. Monoclonal antibodies specific for Shigella flexneri lipopolysaccharides: clones binding to type IV, V, and VI antigens, group 3,4 antigen, and an epitope common to all Shigella flexneri and Shigella dysenteriae type 1 stains. Infect Immun. 1987 Jun;55(6):1412–1420. doi: 10.1128/iai.55.6.1412-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson B. A., Trollfors B., Lagergard T., Taranger J., Bryla D., Otterman G., Cramton T., Yang Y., Reimer C. B., Robbins J. B. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988 May;112(5):695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- Cohen D., Green M. S., Block C., Slepon R., Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991 Feb;29(2):386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Cross A. S., Wegmann A., Germanier R., Sadoff J. C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987 Jul;80(1):51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev B. A., Knirel Y. A., Kochetkov N. K. Somatic antigens of shigella. Structural investigation on the O-specific polysaccharide chain of Shigella dysenteriae type 1 lipopolysaccharide. Eur J Biochem. 1976 Jul 15;66(3):559–566. doi: 10.1111/j.1432-1033.1976.tb10582.x. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Levine M. M., Hornick R. B., Formal S. B. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989 Jun;159(6):1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Kapfer C. Shigella vaccines. Rev Infect Dis. 1989 May-Jun;11 (Suppl 3):S547–S551. doi: 10.1093/clinids/11.supplement_3.s547. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Kent T. H., May H. C., Palmer A., Falkow S., LaBrec E. H. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. J Bacteriol. 1966 Jul;92(1):17–22. doi: 10.1128/jb.92.1.17-22.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON J. E., ASCOLI W., PIERCE V., GUZMAN M. A., MATA L. J. STUDIES OF DIARRHEAL DISEASE IN CENTRAL AMERICA. VI. AN EPIDEMIC OF DIARRHEA IN A GUATEMALAN HIGHLAND VILLAGE, WITH A COMPONENT DUE TO SHIGELLA DYSENTERIAE, TYPE 1. Am J Trop Med Hyg. 1965 May;14:404–411. [PubMed] [Google Scholar]
- Hale T. L., Guerry P., Seid R. C., Jr, Kapfer C., Wingfield M. E., Reaves C. B., Baron L. S., Formal S. B. Expression of lipopolysaccharide O antigen in Escherichia coli K-12 hybrids containing plasmid and chromosomal genes from Shigella dysenteriae 1. Infect Immun. 1984 Nov;46(2):470–475. doi: 10.1128/iai.46.2.470-475.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein H. D., Mills D. F., Outschoorn A. S., Rastogi S. C. The processing and collaborative assay of a reference endotoxin. J Biol Stand. 1983 Oct;11(4):251–260. doi: 10.1016/s0092-1157(83)80013-4. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Petersson K., Katzenellenbogen E., Romanowska E. Structural studies of Shigella flexneri O-antigens. Eur J Biochem. 1978 Nov 2;91(1):279–284. doi: 10.1111/j.1432-1033.1978.tb20963.x. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E. Synthesis of immunogenic oligosaccharide-protein conjugates from the lipopolysaccharide of Neisseria gonorrhoeae P9. J Immunol Methods. 1982;48(2):233–240. doi: 10.1016/0022-1759(82)90197-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Haeggman S., Karlsson K., Phung D. C., Dang D. T. The humoral antibody response to Shigella dysenteriae type 1 infection, as determined by ELISA. Bull World Health Organ. 1984;62(4):597–606. [PMC free article] [PubMed] [Google Scholar]
- McIver J., Grady G. F., Formal S. B. Immunization with Shigella dysenteriae type 1: evaluation of antitoxic immunity in prevention of experimental disease in rhesus monkeys (Macaca mulatta). J Infect Dis. 1977 Sep;136(3):416–421. doi: 10.1093/infdis/136.3.416. [DOI] [PubMed] [Google Scholar]
- Mel D. M., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ. 1965;32(5):647–655. [PMC free article] [PubMed] [Google Scholar]
- Oravec L. S., Lee C. J., Hoppe P. A., Santos C. V. Detection of blood group A-like substance in bacterial and viral vaccines by countercurrent immunoelectrophoresis using Helix pomatia lectin. J Biol Stand. 1984;12(2):159–166. doi: 10.1016/s0092-1157(84)80049-9. [DOI] [PubMed] [Google Scholar]
- Pal S. C. Epidemic bacillary dysentery in West Bengal, India, 1984. Lancet. 1984 Jun 30;1(8392):1462–1462. doi: 10.1016/s0140-6736(84)91948-2. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Greene K. D., Gerber A. R., Tauxe R. V., Vallejo Aguilar O. J., Blake P. A. Shigella dysenteriae type 1 infections in US travellers to Mexico, 1988. Lancet. 1989 Sep 2;2(8662):543–545. doi: 10.1016/s0140-6736(89)90662-4. [DOI] [PubMed] [Google Scholar]
- Raghupathy P., Date A., Shastry J. C., Sudarsanam A., Jadhav M. Haemolytic-uraemic syndrome complicating shigella dystentery in south Indian children. Br Med J. 1978 Jun 10;1(6126):1518–1521. doi: 10.1136/bmj.1.6126.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman M. M., Khan M. M., Aziz K. M., Islam M. S., Kibriya A. K. An outbreak of dysentery caused by Shigella dysenteriae type 1 on a coral island in the Bay of Bengal. J Infect Dis. 1975 Jul;132(1):15–19. doi: 10.1093/infdis/132.1.15. [DOI] [PubMed] [Google Scholar]
- Reed W. P., Albright E. L. Serum factors responsible for killing of Shigella. Immunology. 1974 Jan;26(1):205–215. [PMC free article] [PubMed] [Google Scholar]
- Roy R., Katzenellenbogen E., Jennings H. J. Improved procedures for the conjugation of oligosaccharides to protein by reductive amination. Can J Biochem Cell Biol. 1984 May;62(5):270–275. doi: 10.1139/o84-037. [DOI] [PubMed] [Google Scholar]
- Shahid N. S., Rahaman M. M., Haider K., Banu H., Rahman N. Changing pattern of resistant Shiga bacillus (Shigella dysenteriae type 1) and Shigella flexneri in Bangladesh. J Infect Dis. 1985 Dec;152(6):1114–1119. doi: 10.1093/infdis/152.6.1114. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Santosham M., Reid G. R., Thompson C., Almeido-Hill J., Morell A., deLange G., Ketcham J. K., Callahan E. H. Impaired antibody response to Haemophilus influenzae type b polysaccharide and low IgG2 and IgG4 concentrations in Apache children. N Engl J Med. 1990 Nov 15;323(20):1387–1392. doi: 10.1056/NEJM199011153232005. [DOI] [PubMed] [Google Scholar]
- Sturm S., Jann B., Jann K., Fortnagel P., Timmis K. N. Genetic and biochemical analysis of Shigella dysenteriae 1 O antigen polysaccharide biosynthesis in Escherichia coli K-12: structure and functions of the rfb gene cluster. Microb Pathog. 1986 Jun;1(3):307–324. doi: 10.1016/0882-4010(86)90056-2. [DOI] [PubMed] [Google Scholar]
- Sutton A., Vann W. F., Karpas A. B., Stein K. E., Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985 Oct 10;82(2):215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981 May;32(2):490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Stone A. L., Robbins J. D., Schneerson R., Robbins J. B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Echeverria P., Sethabutr O., Pitarangsi C., Leksomboon U., Blacklow N. R., Rowe B., Gross R., Cross J. Clinical and microbiologic features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J Clin Microbiol. 1988 Jul;26(7):1362–1366. doi: 10.1128/jcm.26.7.1362-1366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Rivera E., Hochstein H. D. Measurements of lipopolysaccharide (endotoxin) in meningococcal protein and polysaccharide preparations for vaccine usage. J Biol Stand. 1989 Jul;17(3):249–258. doi: 10.1016/0092-1157(89)90017-6. [DOI] [PubMed] [Google Scholar]
- Verheul A. F., Braat A. K., Leenhouts J. M., Hoogerhout P., Poolman J. T., Snippe H., Verhoef J. Preparation, characterization, and immunogenicity of meningococcal immunotype L2 and L3,7,9 phosphoethanolamine group-containing oligosaccharide-protein conjugates. Infect Immun. 1991 Mar;59(3):843–851. doi: 10.1128/iai.59.3.843-851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Watanabe H., Nakamura A., Timmis K. N. Small virulence plasmid of Shigella dysenteriae 1 strain W30864 encodes a 41,000-dalton protein involved in formation of specific lipopolysaccharide side chains of serotype 1 isolates. Infect Immun. 1984 Oct;46(1):55–63. doi: 10.1128/iai.46.1.55-63.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Timmis K. N. A small plasmid in Shigella dysenteriae 1 specifies one or more functions essential for O antigen production and bacterial virulence. Infect Immun. 1984 Jan;43(1):391–396. doi: 10.1128/iai.43.1.391-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. B., Marton K. I., Lewis J. N., Friedmann C. T., Gangarosa E. J. Impact in the United States of the Shiga dysentery pandemic of Central America and Mexico: a review of surveillance data through 1972. J Infect Dis. 1974 Feb;129(2):218–223. doi: 10.1093/infdis/129.2.218. [DOI] [PubMed] [Google Scholar]
- Williamson W. A., Greenwood B. M. Impairment of the immune response to vaccination after acute malaria. Lancet. 1978 Jun 24;1(8078):1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]




