Abstract
Hypertension and type II diabetes are co-morbid diseases that lead to the development of nephropathy. Soluble epoxide hydrolase (sEH) inhibitors are reported to provide protection from renal injury. We hypothesized that the sEH inhibitor 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) protects the kidney from the development of nephropathy associated with hypertension and type II diabetes. Hypertension was induced in spontaneously diabetic Goto-Kakizaki rats using angiotensin II and a high salt diet. Hypertensive Goto-Kakizaki rats were treated for two weeks with either AUDA or its vehicle added to drinking water. Mean arterial pressure increased from 118 ± 2 mmHg to 182 ± 20 and 187 ± 6 mmHg for vehicle and AUDA treated hypertensive Goto-Kakizaki rats, respectively. AUDA treatment did not alter blood glucose. Hypertension in Goto-Kakizaki rats resulted in a 17-fold increase in urinary albumin excretion that was decreased with AUDA treatment. Renal histological evaluation determined that AUDA treatment decreased glomerular and tubular damage. In addition, AUDA treatment attenuated macrophage infiltration and inhibited urinary excretion of MCP-1 and kidney cortex MCP-1 gene expression. Taken together, these data provide evidence that sEH inhibition with AUDA attenuates the progression of renal damage associated with hypertension and type II diabetes.
Keywords: diabetes, inflammation, eicosanoids, nephropathy, blood pressure
INTRODUCTION
Hypertension is a major risk factor for the development of nephropathy in patients with type II diabetes [1]. If left untreated, hypertension exacerbates the progression of nephropathy to end stage renal disease (ESRD), which is the leading cause of mortality in patients with type II diabetes. It has been estimated that more than three million people in the United States have hypertension and diabetes and that these disease states are co-morbid [1]. Although the pathological progression of nephropathy is defined clinically, the initiating and propagating factors that lead to the development of nephropathy and its progression to ESRD have yet to be elucidated. A number of metabolic and hemodynamic factors have been identified that could contribute to the decline in renal function. Recent findings have also implicated the involvement of a sub-acute inflammatory component that is characterized by an increase in the infiltration of pro-inflammatory cells into renal tissue and an increase in the transcription of nuclear factor-KB mediated pro-inflammatory genes [2,3]. This inflammatory response can result in structural alterations within the kidney that perturb glomerular function and lead to an increase in glomerular filtration rate all of which occur early in the progression of nephropathy to ESRD.
Epoxygenase metabolites and their regulation by the soluble epoxide hydrolase (sEH) enzyme have been reported to regulate renal blood flow and blood pressure [4-6]. In addition to maintaining renal homeostasis, the epoxides, EETs and epoxide hydrolase inhibition are reported to prevent inflammation by inhibiting the expression of cell adhesion molecules and suppressing leukocyte adhesion [7,8]. Interestingly, increasing epoxygenase metabolite levels or sEH inhibition has been demonstrated to provide cardiovascular and renal protection. Given the renal-protective and anti-inflammatory properties, inhibition of sEH has been identified as a target for the treatment of diseases like hypertension and inflammation [9]. Pharmacological inhibitors of the sEH bind competitively and with high affinity to the catalytic site of the sEH, inhibiting its enzymatic activity [10]. These urea based sEH inhibitors have been modified to allow for oral administration [11]. Thus, we hypothesized that sEH inhibition could protect the kidney from the development of nephropathy associated with hypertension and type II diabetes.
In the present studies, we administered the sEH inhibitor 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) for two weeks in hypertensive Goto-Kakizaki type II diabetic rats. We propose that the protection afforded the kidney results at least in part by inhibiting the inflammatory component of nephropathy. We examined the effects of AUDA, an orally active epoxide hydrolase inhibitor on mean arterial pressure and heart rate. In separate experiments, we investigated the effects of AUDA on renal injury and the infiltration of pro-inflammatory cells into the kidney.
MATERIALS AND METHODS
Animals
The Medical College of Georgia Animal Care and Use Committee approved all animal protocols. Twelve-week-old, male Goto-Kakizaki rats (Taconic, Germantown, NY) were divided into three experimental groups; the first group served as a control and received a normal salt diet and tap water (Goto-Kakizaki control), the second group was made hypertensive and received vehicle treatment, and the third group was made hypertensive and administered AUDA dissolved in the drinking water. Age matched, male Wistar rats (Taconic) were included as a comparison group. Hypertension was induced by continuous infusion of angiotensin II (ANG, 65 ng/min) via an osmotic mini-pump (model 2004, Alzet, Palo Alto, CA). Rats from this group were also fed a high salt diet containing 8% NaCl by weight. AUDA (25 mg/L) was dissolved in a drinking water solution as previously described [12]. Rats from all of the groups were allowed access to food and water ad libitum during the 14-day treatment period.
Measurement of Blood Pressure
Telemetry transmitters (Data Sciences, Inc., St. Paul, MN) were implanted and data collected as described previously [13]. The mean arterial pressure and heart rate were measured once every 5 minutes for the duration of the experimental protocol. Day and night time averages were calculated and plotted. The Biotelemetry Core at the Medical College of Georgia provided assistance with telemetry studies.
Measurement of Urinary Electrolytes, Albumin and MCP-1
Rats were housed in metabolic cages that separate urine from food and feces 24h prior to the completion of the treatment period. The urine was collected in a tube containing 5 mg triphenylphosphine. Urine volumes were measured and the urine aliquoted and stored at −80 °C until analyzed. Concentrations of urinary electrolytes (Na+, Cl−, K+) were measured using ion-selective electrodes (Synchron EL-ISE, Beckman Instruments, Brea, CA). Albumin (Exocell, Inc., Philadelphia, PA) and MCP-1 (BD Biosciences, San Jose, CA) concentrations were measured using enzyme linked immunosorbent assays.
Measurement of Plasma Insulin, Cholesterol and Triglycerides
Whole blood was collected into a heparanized syringe and transferred to a centrifuge tube. The un-coagulated blood was spun for 5 min at 1,000g to sediment red blood cells. The plasma was pipetted off and aliquoted. Aliquots were stored at −80 °C until assayed. Plasma insulin was measured using an enzyme linked immunosorbent assay (Alpco Diagnostics, Windham, NH). Cholesterol and total triglycerides were measured using a colorimetric assay (WAKO Chemicals, Richmond, VA).
Histology and Immunohistochemistry
At the end of the treatment period, kidneys were isolated and perfused with ice-cold physiological salt solution (composition) followed by a 10% formalin solution to fix the kidney tissue. After perfusion, the kidneys were removed, cut and fixed in 10% formalin solution overnight. The kidney sections were embedded in paraffin and cut into 4 μm slices for use in histology and immunohistochemistry protocols. For histology, formalin-fixed paraffin-embedded kidney slices were deparaffinized, re-hydrated and stained with hematoxylin-eosin. Another series were stained using a Masson Trichrome Kit according to the manufacturer's protocol. For immunohistochemistry, deparaffinized, re-hydrated kidney slices were incubated with a 10% hydrogen peroxide solution in methanol to block endogenase peroxidase activity and then blocked with normal goat serum. Kidney slices were then incubated with a primary antibody that recognizes monocytes/macrophages (mouse anti-rat CD68). Sections were incubated with anti mouse secondary antibody conjugated to HRP and visualized using diaminobenzamine chromogen. Slides were counterstained with hematoxylin.
Evaluations of renal damage from hematoxylin-eosin stained sections were performed without the evaluator having knowledge of the treatment groups. Kidney sections were scored using the following numeric scale: 0 = no damage, +1 = very mild, +2 = mild, +3 = moderate, +4 = severe. Evaluation of fibrosis and renal injury were further evaluated in Masson Trichrome stained kidney section. Stained sections were visualized by light microscopy and representative digital images of 5 cortex and 3 medulla areas were obtained for each kidney. To quantify the Masson Trichrome staining, the 8 random images from each kidney were assigned random numbers and scored by three blinded observers on a scale of 0 to 10 for collagen deposition, fibrosis and renal injury. Semiquantitative evaluation of the renal inflammatory cell infiltration was also performed without the evaluator having knowledge of the treatment groups. The numbers of CD68 positive cells were counted from a given area of kidney and the numbers obtained from each treatment group averaged and plotted.
Measurement of AUBA Levels
To confirm AUDA treated groups, urinary levels of the inactive AUDA metabolite 4-(3-adamantan-1-yl-ureido)-butanoic acid (AUBA) was measured in the urine [15]. Briefly, the analytes were separated from other components in the urine by solid phase extraction using a conditioned Oasis-HLB SPE cartridge. The analytes were eluded form the column with ethyl acetate. The ethyl acetate was evaporated under nitrogen and the resulting residue suspended in 50 μl of methanol containing internal standards. The samples were separated using high pressure liquid chromatography. Analyte concentration was determined by comparison to a measured standard curve.
Measurement of Blood Glucose
Non-fasting blood glucose was measured from blood obtained by tail prick. The amount of glucose in the blood was determined using the Accu-chek Advantage blood glucose monitoring system (Roche, Indianapolis, IN).
Real-time Polymerase Chain Reaction (PCR)
Total RNA was prepared from kidney cortex using ultra-pure TRIzol reagent according to the manufacturer's instructions (GIBCO-BRL, Grand Island, NY). Reverse transcription was performed on equal amounts of total RNA (3 μg) using a blend of oligo-dT primers and random hexanucleotide primers to produce a cDNA library for each sample. Real-time PCR reactions were run on an iCycler iQ Real-Time PCR Detection System using iQ Supermix which is optimized for real-time PCR applications (BioRad Laboratories, Inc., Hercules, CA). TaqMan probes (Roche Molecular Systems) and oligonucleotide primers were designed from the published cDNA sequences for MCP-1 and GAPDH using Beacon Designer software (Premier Biosoft International, Palo Alto, CA). Each sample was run in duplicate and the comparative Ct method was used to quantify fold increase compared to controls. Probes and primer sequences were used as follows: MCP-1 probe 5′-FAM-CAC CTG CTG CTA CTC ATT CAC TGG C-BHQ-3′; MCP-1 forward 5′-CAG CCA GAT GCA GTT AAT GC-3′; MCP-1 reverse 5′-GCT TGG TGA CAA ATA CTA CAG C-3′; GAPDH probe 5′-FAM-ACT CCA CGA CAT ACT CAG CAC CAG CA-BHQ-3′; GAPDH forward 5′-CAC GGC AAG TTC AAC GGC-3′; GAPDH reverse 5′-GGT GGT GAA GAC GCC AGT A-3′.
NFκB Transcription Factor Assay
Whole-cell lysates were obtained from kidney cortex using the Nuclear Extract Kit (Active Motif, Carlsbad, CA). Protein concentrations were determined using a bicinchoninic acid protein assay (Pierce, Rockford, IL). Twenty μg of whole-cell extract was used for the determination of NFκB activity using the TransAM NFκB p65 Transcription Factor Assay Kit (Active Motif, Carlsbad, CA). Each of the standards and samples were run in duplicate. In addition to the Jurkat cell nuclear extract provided in the kit as a positive control, HeLa cell whole-cell lysate from cells cultured for 5 min in the presence and in the absence of 10μg/ml TNF-α and 10μM Calyculin A were also run. To ensure NFκB specificity, HeLa whole-cell lysate from cells treated with TNF-α and the whole-cell lysate obtained from experimental groups were run in the presence of the wild-type NFκB consensus oligonucleotide and mutated NFκB consensus oligonucleotide. The wild-type consensus oligonucleotide completely blocked NFκB binding with absorbance that was not different from the blank wells (no NFκB p65 standard). Conversely, the mutated consensus oligonucleotide was without effect on NFκB binding (data not shown). The amount of activated NFκB was normalized per μg of cortical protein used in the assay.
Statistics
All data are presented as mean ± SEM. Statistical significance between experimental groups was determined with an ANOVA. In the event that the associated F ratio indicated that changes occurred, a least significant difference test was used to identify individual differences. P values of 0.05 or less were considered statistically significant.
RESULTS
Urinary excretion of AUDA and AUBA were measured to confirm proper sEH inhibition. AUDA excretion averaged 28 ± 9 ng/d and AUBA excretion averaged 24,049 ± 6,234 mg/d in hypertensive Goto-Kakizaki rats after 14 days of AUDA administration.
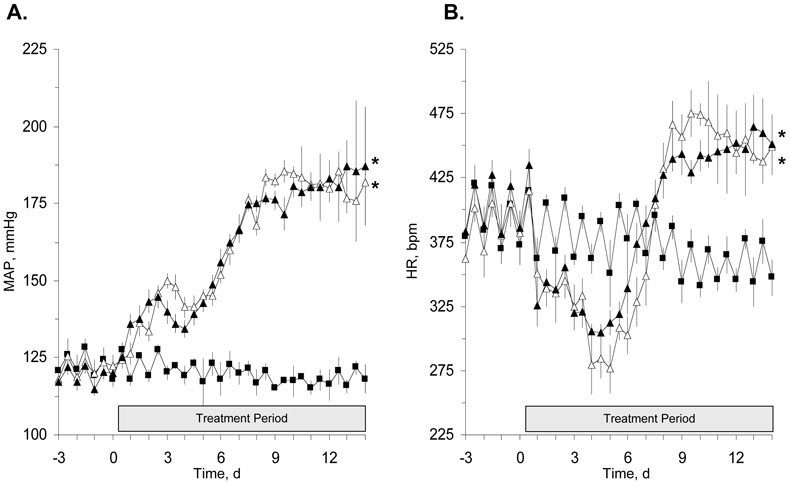
It is well documented that a major risk factor in the development and progression of nephropathy in patients with type II diabetes is hypertension [16,17]. Our laboratory has reported that sEH inhibition lowered blood pressure and provided renal protection in an animal model of hypertension [13,18]. To determine if sEH inhibition lowers blood pressure and/or protects the kidney from the renal damage associated with hypertension in type II diabetes, AUDA was administered to hypertensive Goto-Kakizaki rats. Arterial blood pressure and heart rate are represented graphically in Figures 1A and 1B, respectively. Treatment of hypertensive Goto-Kakizaki rats with AUDA did not lower arterial blood pressure, 182 ± 20 versus 187 ± 6 mmHg for vehicle and AUDA treated hypertensive Goto-Kakizaki rats, respectively. The arterial blood pressure of Goto-Kakizaki control rats measured 118 ± 2 mmHg. AUDA treatment did not alter heart rate in hypertensive Goto-Kakizaki rats. As predicted, hypertension resulted in an initial decrease in the heart rate of Goto-Kakizaki rats with the heart rate reaching its lowest point after five days. Heart rates measured 280 ± 2 bpm for vehicle treated and 307 ± 3 bpm for AUDA treated hypertensive Goto-Kakizaki groups compared to 368± 12 bpm in Goto-Kakizaki controls on the fifth day. After the fifth day, the heart rate of the hypertensive Goto-Kakizaki rats and the AUDA treated hypertensive Goto-Kakizaki rats increased and averaged significantly higher than the Goto-Kakizaki control rats. The heart rates measured 456 ± 4 and 448 ± 3 bpm for vehicle and AUDA treated hypertensive Goto-Kakizaki groups, respectively and 357 ± 4 bpm for the Goto-Kakizaki control group.
Figure 1.
The effect of AUDA on mean arterial pressure (MAP, Panel A) and heart rate (HR, Panel B) in hypertensive Goto-Kakizaki (GK) rats (n=4). Data points represent 12 hour day and night averages of MAP and HR in GK control rats (closed squares) and hypertensive GK rats treated with AUDA (closed triangle) or its vehicle (open triangle). Values represent means ± SEM. *, different from GK control rats, P<0.05.
In separate studies, body weight, urine volumes and urine electrolytes were measured at the end of the two-week treatment period as well as blood glucose, plasma insulin, total cholesterol and triglycerides. These results are summarized in Table 1. As expected, the Goto-Kakizaki control group has elevated non-fasting blood glucose and is consistent with the Goto-Kakizaki rat as a model of type II diabetes. The Goto-Kakizaki control group also has significantly decreased plasma insulin and these data are congruous with impaired insulin secretion and impaired development of pancreatic islet cells both of which are involved in the pathogenesis of diabetes in the Goto-Kakizaki rat [19-21]. Although the Goto-Kakizaki control and hypertensive groups have elevated blood glucose, plasma cholesterol and triglyceride levels, AUDA treatment did not change these levels in hypertensive Goto-Kakizaki rats. Hypertensive Goto-Kakizaki rats demonstrated a significant decrease in body weight and a significant increase in urine volume compared to the Goto-Kakizaki control group. The increased urinary volume was accompanied by an expected increase in sodium and chloride excretion resulting from the angiotensin and high salt diet-induced hypertension. Urinary electrolyte excretion was not altered in the hypertensive Goto-Kakizaki rats administered AUDA.
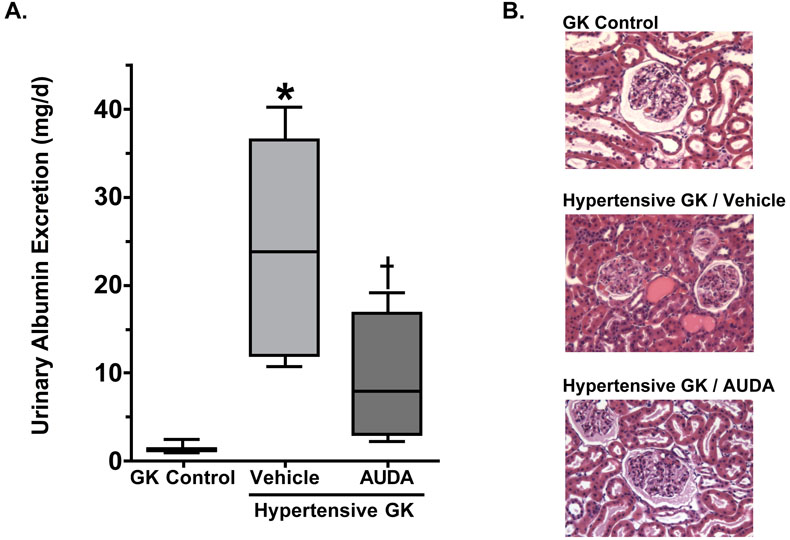
To investigate whether or not soluble epoxide hydrolase inhibition could prevent renal damage associated with hypertension and type II diabetes urinary excretion of albumin was measured, an indicator of renal damage. Urinary albumin excretion averaged 0.9 ± 0.2 mg/d at day 0, 2.5 ± 0.4 mg/d at day 7, and 1.4 ± 0.2 mg/d at day 14 in Goto-Kakizaki controls and at the end of the experimental period this level is comparable to that observed in Wistar rats (1.0 ± 0.4 mg/d). Induction of hypertension in Goto-Kakizaki rats resulted in an increase in urinary albumin excretion at day 7 (8.6 ± 2.6 mg/d) and a 17-fold increase to 22.0 ± 5.0 mg/d at day 14, indicating the development of renal damage Figure 2. AUDA treatment inhibited hypertension-induced increases urinary albumin excretion at day 7 (2.6 ± 1.6 mg/d) and day 14 (6.3 ± 2.6 mg/day).
Figure 2.
The effects of AUDA treatment on (A) urinary albumin excretion and (B) kidney morphology from hypertensive Goto-Kakizaki (GK) rats (n=6-9). *, different from GK control and Hypertensive GK AUDA groups; =, different from GK control group, P<0.05
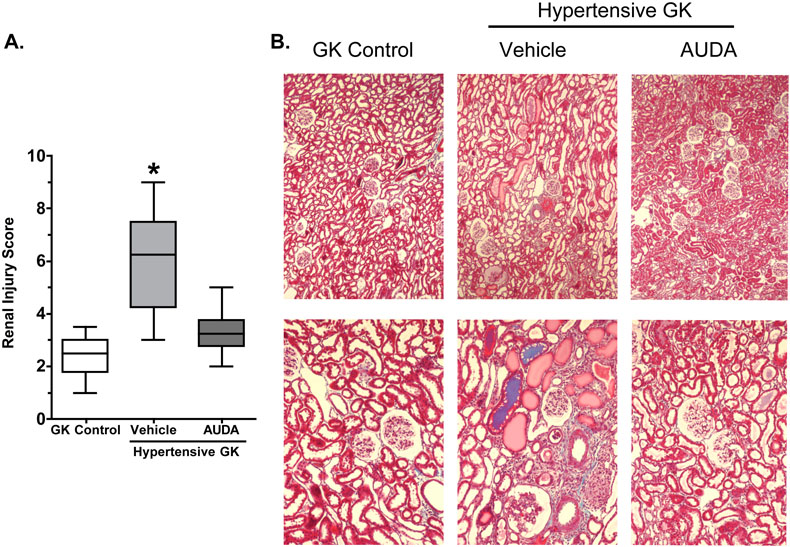
To determine the extent of the renal damage semiquantitative analyses of histological sections were made. Representative sections are provided in Figures 2 and 3. The Goto-Kakizaki control group showed very mild tubular dilation (+1), tubular atrophy (+1) and glomerular hypertrophy (+1). This is comparable to Wistar rats that did not have any damage, sclerosis or necrosis of glomeruli detected in histological sections (data not shown). Hypertensive Goto-Kakizaki rats demonstrated mild to moderate renal injury with increased fibroid necrosis (+3) and hyaline arteriopathy of the interstitial arterioles (+3), increased tubular dilation (+3), tubular atrophy (+2), cast formation (+2) and increased mononuclear cell infiltration (+2). Hypertension did not alter the glomerular hypertrophy present in the Goto-Kakizaki control group. Treatment with AUDA reduced hypertension induced renal injury scores for fibroid necrosis (+1) and hyaline arteriopathy of the interstitial arterioles (+1). AUDA treatment also decreased tubular dilation (+2), atrophy (+1) and cast formation (+1) as well as decreased the score for mononuclear cell infiltration (+1); however, glomerular hypertrophy scores were unchanged. Masson Trichrome staining was evaluated to assess the extent of renal fibrosis and injury. As depicted in Figure 3, there was extensive fibrosis and renal injury in the hypertensive Goto-Kakizaki that was ameliorated by AUDA treatment. Taken together these data provide support for the hypothesis that inhibition of the soluble epoxide hydrolase protects the kidney from renal damage associated with hypertension and type II diabetes independent of any affects on blood pressure.
Figure 3.
The effects of AUDA treatment on (A) the renal fibrosis and injury score and (B) kidney morphology in hypertensive Goto-Kakizaki rats (n=4). *, different from all other groups, P<0.05.
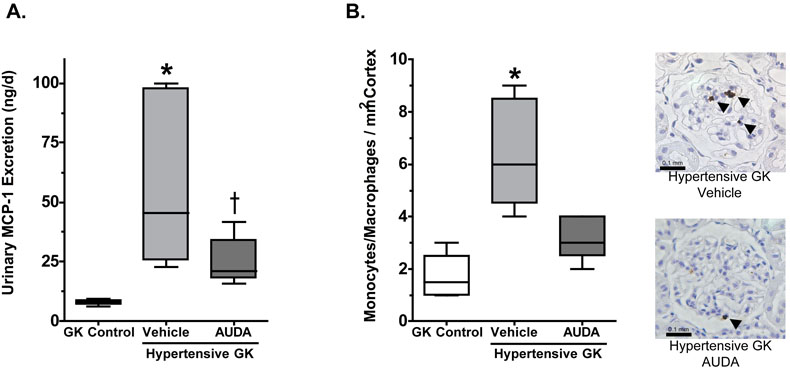
The progression of nephropathy associated with hypertension and type II diabetes involves an inflammatory component that is characterized by increased expression of pro-inflammatory genes and infiltration of pro-inflammatory cells into the kidney interstitium. Recently, sEH inhibition has been identified as a target for the treatment of inflammatory diseases [22,23]. To determine if sEH inhibition decreases the infiltration of pro-inflammatory cells into the kidney, the infiltration of monocyte/macrophages was measured. Goto-Kakizaki control averaged 1.3 ± 0.3 monocytes /mm2 and Wistar rats averaged 1.6 ± 0.4 monocytes /mm2. The number of monocytes/macrophages in the medulla were also determined and found not to be different between any of the treatment groups (data not shown). Inducing hypertension in Goto-Kakizaki rats resulted in a 4-fold increase in monocyte/macrophage infiltration into the kidney cortex (Figure 4). Interestingly, this increase in monocyte/macrophage infiltration corresponds with the measured increase in urinary albumin excretion (Figure 2). Treatment of hypertensive Goto-Kakizaki rats with AUDA significantly attenuated hypertension-induced increases in monocyte/macrophage infiltration into the kidney cortex.
Figure 4.
The effects of AUDA treatment on (A) urinary excretion of MCP-1 and (B) infiltration of pro-inflammatory cells into the kidney cortex of hypertensive Goto-Kakizaki (GK) rats (n=6-7). *, different from GK control and Hypertensive GK AUDA groups; =, different from GK control group, P<0.05
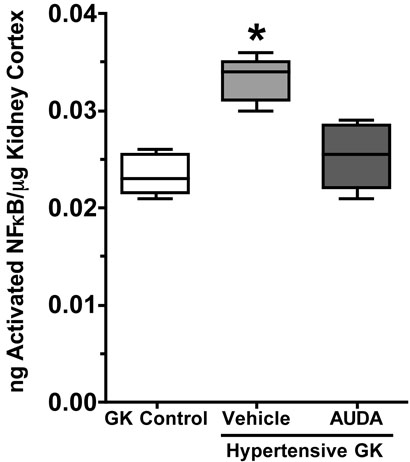
Activation and infiltration of monocytes/macrophages from the bloodstream into the tissue results in part from increased transcription and production of monocyte chemoattractant protein-1 (MCP-1). To determine if the increased infiltration of monocyte/macrophages into the kidney resulted from an increase in MCP-1 protein, urinary MCP-1 excretion was measured. As depicted in Figure 4, the Goto-Kakizaki control group had low levels of MCP-1 in the urine. Urinary excretion of MCP-1 was significantly increased in the hypertensive Goto-Kakizaki group. AUDA treatment attenuated hypertension-induced increases in urinary MCP-1 excretion. These data are in agreement with the infiltration of monocytes/macrophages into the kidney. To determine if the increase in urinary MCP-1 excretion resulted from an increase in MCP-1 transcription kidney cortex was obtained and the amount of MCP-1 mRNA measured by PCR. Cortex from the Goto-Kakizaki control group had low levels of MCP-1 gene expression (2−ΔΔCt = 1.21 ± 0.29). The hypertensive Goto-Kakizaki group demonstrated a 3.4-fold increase in MCP-1 gene expression in the kidney cortex (2−ΔΔCt = 3.57 ± 0.43), which was inhibited to control values with AUDA treatment (2−ΔΔCt = 1.14 ± 0.27). Consistent with the other inflammatory data, renal cortical NFκB activity was significantly higher in hypertensive Goto-Kakizaki group and AUDA treatment reduced NFκB activity in hypertensive Goto-Kakizaki rats (Figure 5). Taken together, these data suggest that inhibition of the soluble epoxide hydrolase inhibits renal damage by inhibiting the infiltration of pro-inflammatory cells into the kidney associated with hypertension and type II diabetes.
Figure 5.
The effects of AUDA treatment on renal NFkB activity in Goto-Kakizaki rats (n=6-8). *, different from all other groups, P<0.05.
DISCUSSION
Hypertension is a major risk factor for patients with type II diabetes. It has been proposed that hypertension and type II diabetes are co-morbid disease states and that the development of hypertension exacerbates the progression of diabetic nephropathy to ESRD. An inflammatory response has been identified that contributes to this progression. This sub-acute inflammatory response is characterized by an increase in the infiltration of pro-inflammatory cells into the kidney and an increase in the expression of pro-inflammatory genes [2,3]. The sEH has been identified as a target for the treatment of hypertension and inflammation. Inhibitors of sEH have been reported to decrease blood pressure in hypertension and decrease hypertension-induced renal damage [13,24]. In addition, sEH inhibitors have demonstrated anti-inflammatory properties and were reported to decrease inflammation-induced tissue damage [22,23]. In the present study, we investigated the hypothesis that sEH inhibition protects the kidney from renal damage associated with hypertension and type II diabetes. Hypertension induced in the spontaneously type II diabetic Goto-Kakizaki rat accelerated the development of renal damage and the infiltration of pro-inflammatory cells into the kidney. AUDA treatment decreased renal damage independent of an effect on blood pressure. In addition, AUDA treatment inhibited the infiltration of pro-inflammatory cells into the kidney and decreased the expression of pro-inflammatory genes.
In the present studies, mean arterial blood pressure increased significantly in Goto-Kakizaki rats infused with angiotensin and fed a high fat diet. The oral administration of AUDA to hypertensive Goto-Kakizaki rats had no effect on mean arterial pressure. This result was of particular interest because the administration of AUDA or other sEH inhibitors were reported to decrease blood pressure in animal models of hypertension [13,18]. Our laboratory has demonstrated that AUDA treatment decreased blood pressure in hypertensive Sprague-Dawley rats. In these experiments hypertension was induced with either angiotensin II infusion or angiotensin II infusion with a high salt diet [25]. The urinary excretion of AUDA averaged 121 ± 61 ng/d for the AUDA treated angiotensin II with high salt diet-induced hypertensive group. In the current study, the urinary excretion of AUDA averaged only 28 ± 9 ng/day for the AUDA treated hypertensive Goto-Kakizaki group [25]. Therefore, it is possible that higher AUDA levels than those attained in the current study are required to elicit effects on blood pressure. Although AUDA did not alter mean arterial pressure, AUDA treatment did have beneficial effects on the hypertensive Goto-Kakizaki rats. Therefore the data presented here suggest that the renal protective effects afforded by AUDA occur via a mechanism that is independent of its effects on blood pressure.
Hypertension induced in the Goto-Kakizaki rat resulted in an increase in blood pressure and was accompanied by a measured increase in heart rate during the second week. It was reported that angiotensin II and/or salt sensitive hypertension resulted in a transient decrease in heart rate that returns to control levels by the end of the first week [25]. Therefore, the ability to control heart rate in the spontaneously type II diabetic Goto-Kakizaki rat after the induction of hypertension using angiotensin II and high salt diet is not the same as rats with hypertension alone. The specific contribution of increased heart rate to the development of renal damage in this animal model of hypertension and type II diabetes is not known.
The progression of renal damage in the spontaneously diabetic Goto-Kakizaki rats was accelerated by hypertension. This was evidenced by and increase in urinary albumin excretion, an indicator of renal damage. In addition, hypertension induced morphological changes in the kidney that are characteristic to the development of nephropathy such as tubular dilation and fibroid necrosis of interstitial arterioles. Similar changes were observed in other models of hypertension induced in the Goto-Kakizaki rat. Salt-sensitive hypertension resulted in a 50% increase in albuminuria and administration of deoxycorticosterone acetate salt to Goto-Kakizaki rats, a model of mineralcorticoid-induced experimental hypertension, induced a 4-fold increase in proteinuria [26,27]. Likewise, heminephrectomized Goto-Kakizaki rats have increased macrophage infiltration and accelerated renal damage [28]. Treatment of hypertensive Goto-Kakizaki rats with AUDA inhibited hypertension-induced increases in albumin excretion and prevented the development of many of the morphological changes induced by hypertension. These renal protective effects resulting from epoxide hydrolase inhibition are consistent with data generated in our laboratory using in vivo models of hypertension. Zhao et al. reported that chronic administration of the epoxide hydrolase inhibitor 1-cyclohexyl-3-dodecylurea (CDU) to angiotensin II-induced hypertensive Sprague-Dawley rats decreased urinary albumin excretion and protected the kidney from hypertension-induced damage [13]. Similarly, it was reported that AUDA, decreased urinary albumin excretion and renal damage in both angiotensin II-induced and angiotensin II-induced salt-sensitive hypertensive Sprague-Dawley rats [25]. Taken together, these data provide support for the hypothesis that AUDA protects the kidney from damage associated with hypertension and type II diabetes.
An inflammatory component has been identified that contributes to the progression of nephropathy to end stage renal disease. This sub-acute inflammatory component is characterized by an increase in the infiltration of inflammatory cells into the kidney and an increase in the expression of pro-inflammatory genes. In the present study, we demonstrated that hypertension exacerbated monocyte/macrophage infiltration in the Goto-Kakizaki kidney. This increased infiltration of inflammatory cells into the kidney cortex was accompanied by an increase in urinary MCP-1 excretion and an increase in MCP-1 gene expression in the kidney cortex. This hypertension-induced increase in the sub-acute inflammatory response corresponds with the observed increase in urinary albumin excretion and renal morphological damage. These data are consistent with renal damage that occurs in other experimental models of hypertension and heminephrectomy in the Goto-Kakizaki rat [26-28]. Cheng et al. reported an increase in monocyte/macrophage infiltration into the kidney with a corresponding increase in immunostaining for the intracellular adhesion molecule-1 in Goto-Kakizaki rats with salt-sensitive hypertension [26]. In addition, administration of deoxycorticosterone acetate salt to Goto-Kakizaki rats resulted in an increase in macrophage infiltration into the kidney and was accompanied by an increase in renal immunohistochemical staining for MCP-1 [28]. Interestingly, treatment of hypertensive Goto-Kakizaki rats with AUDA inhibited the infiltration of monocyte/macrophages into the kidney and decreased gene expression and urinary excretion of MCP-1. Taken together, these data suggest that the development of hypertension in an animal model of type II diabetes exacerbates the progression of renal injury at least in part by inducing a sub-acute inflammatory response and that this response can be attenuated with AUDA treatment.
The sEH enzyme has been identified as a target for the treatment of hypertension and inflammation [9]. In animal models of hypertension, sEH inhibitors are reported to decrease blood pressure and thereby inhibit hypertension-induced renal damage [13,18]. The sEH inhibitors 1-cyclohexyl-3-dodecylurea and AUDA lowered blood pressure in animal models of hypertension and reduced hypertension-induced renal damage [13]. In animal models of systemic inflammation sEH inhibitors are reported to decrease the infiltration of pro-inflammatory cells into tissue and decrease the expression of pro-inflammatory genes. The sEH inhibitor 12-(3-adamantane-1-yl-ureido)-dodecanoic acid n-butyl ester (AUDA-nBE), was reported to inhibit tobacco smoke-induced lung inflammation [22]. In addition, the sEH inhibitors AUDA-nBE and 1-adamantan-3-(5-(2-(2-ethyl-ethoxy)ethoxy)pentyl)urea decreased liposaccharide-induced increases in plasma concentrations of pro-inflammatory cytokines like TNF-alpha, IL-6 and MCP-5 in mice [23]. In these animal models, the protective effects of the sEH inhibitors are attributed to a decrease in EET metabolism. Although increased epoxides is the likely mechanism, it has been reported that AUDA in addition to sEH inhibition can activate the peroxisome proliferator-activated receptor-α (PPAR-α); however the concentration of AUDA required for PPAR-α activation is much higher than the concentrations obtained in the current study [29]. Also, PPAR-α activation would be expected to decrease triglyceride levels, which were unchanged in AUDA treated hypertensive Goto-Kakizaki rats. sEH inhibitors have also been reported to increase the incorporation and retention of the EETs into endothelial phospholipids and enhance the shuttling of the EETs into alternate metabolic pathways [30-32]. The contribution of these alternate actions of the sEH inhibitors in the current study are not known and therefore might contribute to the renal-protective effects observed.
In the present study, we demonstrated that hypertension, induced in a model of type II diabetes, exacerbates the development of renal damage. In addition, we provide evidence that this progression involves a sub-acute inflammatory response. We demonstrated that sEH inhibition protects the kidney from the development of renal damage independent of any effects on blood pressure. A possible mechanism by which AUDA treatment attenuates the development of renal damage in this model of hypertension and type II diabetes is via inhibition of the sub-acute inflammatory response. Taken together, the data presented provides support for the hypothesis inhibition of the epoxide hydrolase ameliorates the inflammatory component of nephropathy associated with hypertension and type II diabetes.
Acknowledgments
Sources of support: AHA Established Investigator Award, AHA Southeast Affiliate Postdoctoral Fellow, National Institutes of Health Grants HL-59699 and HL-74167
REFERENCES
- 1.USRDS: the United States Renal Data System Am. J. Kidney Dis. 2003;42:1–230. [PubMed] [Google Scholar]
- 2.Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. NF-{kappa}B activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol. Dial. Transplant. 2004;10:2505–2512. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 3.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 4.Imig JD. Eicosanoid regulation of the renal vasculature. Am. J. Physiol. Renal Physiol. 2000;279:F965–981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Falck JR. The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochem. Biophys. Res. Commun. 2001;285:571–576. doi: 10.1006/bbrc.2001.5167. [DOI] [PubMed] [Google Scholar]
- 6.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 7.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol. Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- 9.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am. J. Physiol. Renal Physiol. 2005;289:F496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 10.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc. Natl. Acad. Sci. U S A. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J. Med. Chem. 2004;47:2110–2122. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 12.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J. Am. Soc. Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 14.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/ MS. J. Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Morisseau C, Newman JW, Hammock BD. In vitro metabolism of the mammalian soluble epoxide hydrolase inhibitor, 1-cyclohexyl-3-dodecylurea. Drug Metab. Dispos. 2003;31:846–853. doi: 10.1124/dmd.31.7.846. [DOI] [PubMed] [Google Scholar]
- 16.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension. 1995;26:869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 17.Viberti GC, Earle K. Predisposition to essential hypertension and the development of diabetic nephropathy. J. Am. Soc. Nephrol. 1992;3:S27–S33. doi: 10.1681/ASN.V34s27. [DOI] [PubMed] [Google Scholar]
- 18.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 19.Movassat J, Saulnier C, Serradas P, Portha B. Impaired development of pancreatic beta-cell mass is a primary event during the progression to diabetes in the GK rat. Diabetologia. 1997;40:916–925. doi: 10.1007/s001250050768. [DOI] [PubMed] [Google Scholar]
- 20.Picarel-Blanchot F, Berthelier C, Bailbe D, Portha B. Impaired insulin secretion and excessive hepatic glucose production are both early events in the diabetic GK rat. Am. J. Physiol. 1996;271:E755–E762. doi: 10.1152/ajpendo.1996.271.4.E755. [DOI] [PubMed] [Google Scholar]
- 21.Kim CS, Sohn EJ, Kim YS, Jung DH, Jang DS, Lee YM, Kim DH, Kim JS. Effects of KIOM-79 on hyperglycemia and diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. J. Ethnopharmacol. 2007;111:240–247. doi: 10.1016/j.jep.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc. Natl. Acad. Sci. U S A. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. U S A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 25.Imig JD, Zhao X, Zharis C, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An Orally Active Epoxide Hydrolase Inhibitor Lowers Blood Pressure and Provides Renal Protection in Salt-Sensitive Hypertension. Hypertension. 2005;46:1–7. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC, Mervaala EM. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension. 2001;37:433–439. doi: 10.1161/01.hyp.37.2.433. [DOI] [PubMed] [Google Scholar]
- 27.Tefescu A, Kanazawa M, Ishida A, Lu H, Saski Y, Ootaka T, Sato T, Kohzuki M. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J. Hypertens. 2008;26:312–321. doi: 10.1097/HJH.0b013e3282f2450b. [DOI] [PubMed] [Google Scholar]
- 28.Janssen U, Riley SG, Vassiliadou A, Floege J, Phillips AO. Hypertension superimposed on type II diabetes in Goto Kakizaki rats induces progressive nephropathy. Kidney Int. 2003;63:2162–2170. doi: 10.1046/j.1523-1755.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 29.Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, Liu Y, Shyy JY, Hammock BD, Spector AA. Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J. Pharmacol. Exp. Therap. 2005;314:260–270. doi: 10.1124/jpet.105.085605. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J. Biol. Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am. J. Physiol. Heart Circ. Physiol. 1999;277:H2098–H2108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- 32.Imig JD. Eicosanoids and renal vascular function in diseaes. Cli. Sci. 2006;111:21–34. doi: 10.1042/CS20050251. [DOI] [PubMed] [Google Scholar]