Abstract
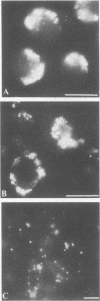
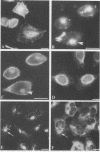
Immunofluorescence was used to examine the distribution of Chlamydia trachomatis serovars L2 and E, F-actin, and clathrin in infected McCoy and HeLa cells. After incubation at 4 degrees C, C. trachomatis serovar L2 was randomly distributed on the McCoy cell surface. After a temperature shift to 37 degrees C, chlamydiae redistributed, within 30 min, to one local aggregate in the central or perinuclear region of individual cells. About 90% of these aggregated chlamydiae were intracellularly localized, but some remained randomly distributed on the cell surface. Similar results were obtained with HeLa cells and C. trachomatis serovar E, except that the redistribution was slower in HeLa cells than in McCoy cells and fewer cells infected with serovar E exhibited a local aggregate than those infected with serovar L2. Cytochalasin D inhibited more than 90% of this local aggregation. Instead, in cytochalasin D-treated cells, the entry of chlamydiae was inhibited and the organisms became localized on the cell surface in a peripheral local aggregate that distributed in a manner similar to that of phalloidin-stained actin. In a double immunofluorescence assay, F-actin and clathrin aggregated correspondingly in time and position with central or perinuclear aggregation of chlamydiae. These results indicate that polymerized actin and clathrin participate in a rapid redistribution of chlamydiae to an intracellular aggregate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Pearce J. H. Modulation by centrifugation of cell susceptibility to chlamydial infection. J Gen Microbiol. 1979 Mar;111(1):87–92. doi: 10.1099/00221287-111-1-87. [DOI] [PubMed] [Google Scholar]
- Blyth W. A., Taverne J. Some consequences of the multiple infection of cell cultures by TRIC organisms. J Hyg (Lond) 1972 Mar;70(1):33–37. doi: 10.1017/s0022172400022063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S., Richmond S. J., Yates P. S. The effect of Chlamydia trachomatis infection on the host cell cytoskeleton and membrane compartments. J Gen Microbiol. 1989 Sep;135(9):2379–2386. doi: 10.1099/00221287-135-9-2379. [DOI] [PubMed] [Google Scholar]
- Clerc P. L., Sansonetti P. J. Evidence for clathrin mobilization during directed phagocytosis of Shigella flexneri by HEp2 cells. Microb Pathog. 1989 Nov;7(5):329–336. doi: 10.1016/0882-4010(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B. Inhibition of phagolysosome fusion is localized to Chlamydia psittaci-laden vacuoles. Infect Immun. 1981 May;32(2):889–896. doi: 10.1128/iai.32.2.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Gregory W. W., Byrne G. I., Gardner M., Moulder J. W. Cytochalasin B does not inhibit ingestion of Chlamydia psittaci by mouse fibroblasts (L cells) and mouse peritoneal macrophages. Infect Immun. 1979 Jul;25(1):463–466. doi: 10.1128/iai.25.1.463-466.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Davis C. H., Choong J., Wyrick P. B. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect Immun. 1988 Jun;56(6):1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Wyrick P. B. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect Immun. 1986 Dec;54(3):855–863. doi: 10.1128/iai.54.3.855-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa G. Y., Trevithick J. R., Bechberger J., Blair D. G. Cytochalasin D induces the capping of both leukaemia viral proteins and actin in infected cells. Nature. 1978 Aug 24;274(5673):808–809. doi: 10.1038/274808a0. [DOI] [PubMed] [Google Scholar]
- Prain C. J., Pearce J. H. Ultrastructural studies on the intracellular fate of Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and Chlamydia trachomatis (strain lymphogranuloma venereum 434): modulation of intracellular events and relationship with endocytic mechanism. J Gen Microbiol. 1989 Jul;135(7):2107–2123. doi: 10.1099/00221287-135-7-2107. [DOI] [PubMed] [Google Scholar]
- Reynolds D. J., Pearce J. H. Characterization of the cytochalasin D-resistant (pinocytic) mechanisms of endocytosis utilized by chlamydiae. Infect Immun. 1990 Oct;58(10):3208–3216. doi: 10.1128/iai.58.10.3208-3216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderhof J. C., Barnes R. C. Fusion of inclusions following superinfection of HeLa cells by two serovars of Chlamydia trachomatis. Infect Immun. 1989 Oct;57(10):3189–3193. doi: 10.1128/iai.57.10.3189-3193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist K. G., Ehrnst A. Cytoskeletal control of surface membrane mobility. Nature. 1976 Nov 18;264(5583):226–231. doi: 10.1038/264226a0. [DOI] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Physicochemical surface properties of elementary bodies from different serotypes of chlamydia trachomatis and their interaction with mouse fibroblasts. Infect Immun. 1982 Jun;36(3):893–899. doi: 10.1128/iai.36.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Prevost M. C., Hellio R., Sansonetti P. J. Stress fiber-based movement of Shigella flexneri within cells. Infect Immun. 1991 May;59(5):1723–1732. doi: 10.1128/iai.59.5.1723-1732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Choong J., Davis C. H., Knight S. T., Royal M. O., Maslow A. S., Bagnell C. R. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989 Aug;57(8):2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]