Abstract
Secondary lymphoid organs develop during embryogenesis or in the first few weeks after birth according to a highly coordinated series of interactions between newly emerging hematopoietic cells and immature mesenchymal or stromal cells. These interactions are orchestrated by homeostatic chemokines, cytokines and growth factors that attract hematopoietic cells to sites of future lymphoid organ development and promote their survival and differentiation. In turn, lymphotoxin-expressing hematopoietic cells trigger the differentiation of stromal and endothelial cells that make up the scaffolding of secondary lymphoid organs. Lymphotoxin signaling also maintains the expression of adhesion molecules and chemokines that govern the ultimate structure and function of secondary lymphoid organs. Here we describe the current paradigm of secondary lymphoid organ development and discuss the subtle differences in the timing, molecular interactions and cell types involved in the development of each secondary lymphoid organ.
Keywords: lymphotoxin, homeostatic chemokine, lymphoid tissue inducer
Summary points
1. The development of secondary lymphoid organs is initiated during embryogenesis by interactions between hematopoietic cells (CD4+CD3−IL-7Rα+ α 4β7+ LTi cells and CD4−CD3−IL-7Rα−CD11c+ LTin cells) that arise in the fetal liver and mesenchymal cells that are located at sites of future lymphoid organ development.
2. The development of LTi cells from precursors in the fetal liver and their local differentiation into LTαβ-expressing cells requires the activities of cytokines, such as TRANCE or IL-7. IL-7 seems to be most important for the differentiation of LTi cells at mucosal sites, such as the Peyer’s patches, while TRANCE is most important at sites of peripheral lymph node development.
3. LTi cells are attracted to sites of lymphoid organ development by homeostatic chemokines, including CXCL13, CCL19 and CCL21. Like TRANCE and IL-7, these chemokines also maintain surface LTαβ expression on LTi cells.
4. The lymphotoxin signalling pathway plays a central role in the development of secondary lymphoid organs due to its ability to trigger mesenchymal cell differentiation, elicit homeostatic chemokine expression and to promote the differentiation of HEVs, stromal cells and dendritic cells.
5. Signalling through the LTβR initiates both the canonical and alternative NFκB signalling pathways.
6. The vascular addressins, MAdCAM and PNAd are sequentially expressed during lymph node development so that LTi cells and mature lymphocytes are recruited in a temporally ordered fashion.
7. CD4+CD3− cells can be found in adults and seem to have a function in facilitating follicular T cell responses. However, the lineage relationship between embryonic CD4+CD3− LTi cells and those found in adults remains uncertain.
8. The developmental pathways for peripheral lymph nodes, mesenteric lymph nodes and Peyer’s patches differ in their requirements for chemokines, cytokines and growth factors. However, the pathways that govern the development of ILFs and NALT are dramatically different than those that govern conventional lymph nodes and Peyer’s patches.
Future issues
1. What is the relationship between CD4+CD3−IL-7Rα+ LTi cells and CD4−CD3−IL-7Rα−CD11c+ LTin cells?
2. Does each secondary lymphoid organ use slightly different populations of LTi and LTo cells for their development and does this differential utilization of cell types explain why each lymphoid organ uses subtly different combinations of chemokines, growth factors and cytokines for their development?
3. Other than the lymphotoxin signalling pathway, what pathways are used by LTi cells to trigger the differentiation of mesenchymal cells and initiate secondary lymphoid organ development?
4. Why is the development of most secondary lymphoid organs (lymph nodes, Peyer’s patches and NALT) restricted to embryogenesis, when the development of ILFs can occur in adults?
5. Why does the development of NALT occur in the absence of lymphotoxin signalling and what pathways substitute for lymphotoxin signalling in this process?
Introduction
Primary immune responses are initiated in secondary lymphoid organs, including spleen, regional lymph nodes, Peyer’s patches, Isolated Lymphoid Follicles (ILFs), tonsils and Nasal Associated Lymphoid Tissue (NALT). These tissues are situated throughout the body at strategic sites where antigens from pathogens are most likely to be encountered. Regional lymph nodes are found along lymphatic vessels that collect antigen and antigen presenting cells from non-lymphoid organs, whereas mucosal lymphoid organs, such as Peyer’s patches, ILFs, tonsils and NALT, lack afferent lymphatics and acquire antigen directly across the mucosal epithelium. The spleen is also a secondary lymphoid organ and has evolved a unique structure to sample blood-borne antigens. All of these secondary lymphoid organs have specialized architecture and microenvironments that promote the controlled interactions of immune cells in order to elicit a rapid and appropriate immune response to infectious agents (reviewed in (1–3)).
Most infectious agents gain entry to the body via the skin or mucosal epithelium, which comprise an extremely large surface area. Moreover, many infectious agents target non-lymphoid tissues and organs to complete their life cycle. Since the frequency of naïve T or B lymphocytes specific for particular epitopes is in the order of 1 in 105–106, it would be very difficult for these few cells to monitor every inch of each peripheral tissue and organ for foreign antigens and pathogens. This problem is elegantly solved by secondary lymphoid organs, which recruit naïve lymphocytes from the blood and attract activated, antigen-bearing antigen presenting cells from regional tissues. In essence, lymphocytes recirculate from lymph node to lymph node and allow antigen to be delivered to them. As a result, the process of immune surveillance is much more efficient and adaptive immune responses to infectious agents are initiated more quickly (reviewed in (1, 4, 5)).
Secondary lymphoid organs develop during embryogenesis or in the early post-natal period. This process occurs independently of antigen or pathogen recognition at pre-determined sites throughout the body as a result of complex interactions between various hematopoietic, mesenchymal and endothelial cells. Many, but by no means all, of the cellular and molecular interactions that are involved in secondary lymphoid organ development are understood and some of these mechanisms are also involved in the maintenance of secondary lymphoid organ architecture in adults as well as the function of these organs during immune responses (previously reviewed in (6–11)). Moreover, similar mechanisms govern the formation of tertiary lymphoid tissues, which develop in adults at sites of persistent infection or chronic inflammation. Since the formation and function of tertiary lymphoid organs has been recently reviewed (12, 13), this review will focus exclusively on the pre-programmed organogenesis of secondary lymphoid organs.
Early cellular interactions in Peyer’s patch development
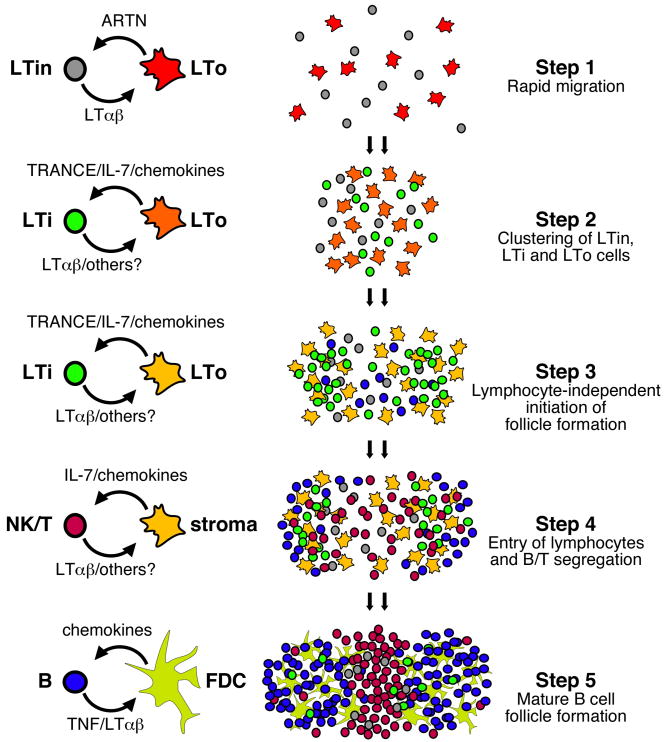
Many of the cellular interactions that initiate secondary lymphoid organ development are well characterized, particularly with regards to Peyer’s patch development (11). Hematopoietic cells derived from precursors in the fetal liver first begin to colonize the gut between days E12.5 and E15.5 (14, 15). Although these cells are evenly distributed throughout the gut on day E15.5, they rapidly form clusters at sites of Peyer’s patch development (16). This is step 1 as shown in Figure 1. These hematopoietic cells include a population of CD4+CD3−IL-7Rα+c-kit+ Lymphoid Tissue inducer (LTi) cells as well as a population of CD4−CD3−IL-7Rα−c-kit+CD11b+CD11c+ Lymphoid Tissue initiator (LTin) cells (16, 17). Both the LTin and LTi cells cluster together with non-hematopoietic VCAM-1+ICAM-1+ Lymphoid Tissue organizer (LTo) cells on the anti-mesenteric side of the small intestine and form the primitive anlagen of the Peyer’s patch (15, 16).
Figure 1. General scheme of secondary lymphoid organ development.
As noted in the text of the review, the details in the developmental pathways of Peyer’s patches and individual lymph nodes are often slightly different. Therefore, this scheme incorporates elements of both Peyer’s patch and lymph node development and outlines major steps that are likely to be in common in these pathways. Importantly, this scheme implies several events that are purely speculative. For example, it is unknown whether LTo cells are the direct precursors of mature stromal cells and follicular dendritic cells (FDCs). It is also unclear whether LTi cells and LTin cells are maintained in mature secondary lymphoid organs in adults.
The available evidence suggests that both LTin and LTi cells are important for the initial stages of Peyer’s patch development. For example, mice depleted of CD11c+ cells during embryogenesis develop fewer Peyer’s patches and mice lacking the tyrosine kinase receptor, RET, which is expressed by LTin cells, completely lack Peyer’s patch anlagen (16). Interestingly, Ret−/− mice have normal numbers of LTin and LTi cells, although these cells do not aggregate and fail to trigger the differentiation of VCAM-1+ICAM-1+ LTo cells (16). One of the ligands for RET, ARTN, is produced by non-hematopoietic VCAM-1+ cells - probably LTo cells, and promotes the clustering of LTin and LTi cells at sites of Peyer’s patch development. In fact, the ectopic application of ARTN to embryonic gut tissue leads to the clustering of both LTin and LTi cells, the local appearance of VCAM-1+ cells and the formation of an ectopic Peyer’s patch anlagen (16).
LTi cells are also important for secondary lymphoid organ development, as mice deficient for the transcription factor, Id2 (18), and mice deficient for the nuclear hormone receptor, RORγ (19) fail to develop IL-7Rα+CD4+CD3− LTi cells and consequently lack lymph nodes and Peyer’s patches (18, 19). Furthermore, the adoptive transfer of purified LTi cells from normal mice into neonatal CXCR5−/− recipients promotes the development of Peyer’s patches (20), and the adoptive transfer of IL-7R3+CD4+CD3− cells into neonatal Id2−/− mice induces the development of Nasal Associated Lymphoid Tissue (NALT) (21). Mice lacking the transcription factor Ikaros also fail to develop lymph nodes (22, 23), a fact that is attributed to the loss of LTi cells. However, given the defects in the dendritic cell lineage in Ikaros mutant mice (24), it is possible that the development of LTin cells is also compromised in these animals and may contribute to the loss of lymphoid organs. Additional evidence for the involvement of LTi cells in lymphoid organ development comes from transgenic mice that over-express IL-7 (25). These mice have abnormally high numbers of LTi cells and develop a much higher number of lymph nodes and Peyer’s patches than normal mice (25). However, IL-7 by itself is not sufficient to drive the development of these lymphoid organs, since IL-7 transgenic mice crossed to Rorc−/− mice fail to produce LTi cells and do not develop any lymph nodes or Peyer’s patches (25). Thus, IL-7Rα+CD4+CD3− cells appear to be essential for lymphoid organ development.
Despite the essential roles that LTin and LTi cells play in secondary lymphoid organ development, the lineage relationship between these cell types remains obscure. LTin cells seem to have some characteristics of dendritic cells, including CD11c, CD11b and MHC class II expression. However, they lack DEC205 and instead express NK1.1 and Gr-1 (16). Importantly, they lack IL-7Rα, which is important for the expansion of LTi precursors and for the expression of LTαβ on LTi cells, particularly at mucosal sites. On the other hand, LTi cells are not easily categorized into a particular lineage. They arise from common lymphoid progenitors in the fetal liver (26), but lack any features of B or T lymphocytes. Under the appropriate conditions, they mature into NK-like cells and CD11c+ dendritic cells (27), suggesting that they may give rise to CD11c+ LTin cells. However, it is currently unclear whether the development of LTin cells also requires RORγ or Id2, which would provide better evidence that the two populations are related.
Early events in lymph node development
The early stages of lymph node development are slightly different that those of Peyer’s patch development. Unlike Peyer’s patches, lymph nodes are encapsulated by lymphatic endothelium and their development occurs concurrently with the process of lymphatic vascularization. As first shown by ink injections and micro-dissection, lymph sacs form from endothelial cells, which bud from the veins during early development (28, 29). These primitive lymph sacs then form buds that branch and form the lymphatic network. This process is controlled by the transcription factor, Prox1, which is expressed exclusively by lymphatic endothelial cells (30) and is essential for the formation of lymphatic vessels (31, 32). In fact, the early lymph node anlagen is bordered by a mix of endothelial cells that have characteristics of both lymphatic and blood vascular endothelial cells (33). Analogous to what happens in developing Peyer’s patches, CD4+CD3−IL-7Rα+ LTi cells aggregate near endothelial cells at sites of future lymph node development and trigger the differentiation of VCAM-1+ICAM-1+ LTo cells to form the primitive lymph node anlagen (34). Although CD11c+ cells are also observed in developing lymph node anlagen (34), it is currently unclear whether these cells are the equivalent of CD11c+ LTin cells or whether RET/ARTN interactions are also important for the development of lymph nodes.
Differential role of TRANCE and IL-7 in Peyer’s patch and lymph node formation
The differences in Peyer’s patch and lymph node organogenesis are also illustrated by the differential roles of the TNF family member TRANCE and the cytokine IL-7 in these processes (34, 35). TRANCE (also known as OPGL/ODF/RANKL/TNFSF11), its receptor, RANK (also known as TRANCE-R/ODFR/OFE) and a critical component of the TRANCE signalling pathway, TRAF6, are all required for the formation of lymph nodes, but not Peyer’s patches or NALT (35–38). Although Trance−/− mice do develop some cervical lymph nodes, those that form are small and poorly populated (35, 36). Moreover, Trance−/− mice have relatively few LTi cells and those that are present fail to properly induce the differentiation of mesenchymal cells (35). TRANCE is expressed by mesenchymal LTo cells in sites of lymph node development and functions to promote the differentiation and survival of LTi cells and to upregulate LTαβ on their surface (33, 34). In turn, the ligation of the LTβR on local mesenchymal cells triggers their differentiation and promotes the expression of CCL19, CCL21 and CXCL13 as well as VCAM-1 and ICAM-1 (34, 39). This is step 2 as shown in Figure 1. These chemokines and adhesion molecules promote LTi clustering and continue to recruit newly emerging lymphocytes to the developing lymph nodes. Therefore, in the absence of TRANCE, VCAM-1+ICAM-1+ cells fail to appear in the lymph node anlagen and lymph nodes fail to complete their program of development.
On the other hand, IL-7Rα, IL-2Rγ and JAK3 are required for the organogenesis of Peyer’s patches, but not lymph nodes (14, 15). This was initially surprising, since the LTi cells in developing lymph nodes express IL-7Rα just like the LTi cells in developing Peyer’s patches (34). In fact, LTi cells in developing lymph nodes are capable of responding to IL-7, as the ectopic application of IL-7 to developing Traf6−/− embryos (which cannot signal through TRANCE) promotes the development of lymph nodes (34). Like TRANCE, IL-7 upregulates LTαβ on the surface of LTi cells (40), which leads to the expression of chemokines and adhesion molecules by LTo cells. Other cytokines that signal through the IL-2Rγ chain, such as IL-4, are also capable of triggering LTαβ expression on LTi cells. However, there is no evidence that these cytokines are involved in secondary lymphoid organ development, probably because these cytokines are not expressed in the developing embryo. Thus, TRANCE and IL-7 perform similar functions and the reason that TRANCE is required for lymph node development and IL-7R is required for Peyer’s patch development is due to differential expression of these cytokines in lymph nodes and Peyer’s patches.
Although these data would suggest that the role for IL-7 in lymph node genesis is minimal, other data demonstrate that mice doubly deficient in CXCL13 and IL-7Rα completely fail to form lymph nodes (40), including mesenteric lymph nodes, which are normally present in Cxcl13−/− mice (40, 41). Moreover, the transgenic over-expression of IL-7 promotes the development of extra lymph nodes as a result of much higher numbers of LTi cells (25). Thus, IL-7 probably also plays a role in the expansion or development of LTi cells from common lymphoid progenitors as well as being a critical inducer of LTαβ expression in some locations.
The counterparts to LTi cells in the development of secondary lymphoid organs are LTo cells, which are are mesenchymal in origin and form the stromal cell matrix of the developing lymphoid organ (14, 33, 39, 42). Just as there seem to be multiple types of hematopoietic cells that trigger lymphoid organ development, there are also multiple types of mesenchymal LTo cells, with different expression patterns of adhesion molecules, cytokines and receptors (43). In fact, the VCAM-1+ICAM-1+ LTo population can be divided into VCAM-1intICAM-1int and VCAM-1hiICAM-1hi populations (33). Whereas both of these populations are present in peripheral and mesenteric lymph nodes, the frequency of VCAM-1intICAM-1int cells is dramatically lower in peripheral lymph nodes (33). Differences between these populations are also found in developing Peyer’s patches and mesenteric lymph nodes. In fact, the LTo cells found at sites of mesenteric lymph node and Peyer’s patch development express very different gene programs (43). For example, LTo cells at sites of mesenteric lymph node development express much higher levels of TGFβ and stem cell factor (the ligand for c-kit), whereas LTo cells at sites of Peyer’s patch development express higher levels of IL-6, TRANCE, CXCL1, CCL7 and CCL11 (43). These differences in gene expression may provide an explanation as to why various lymphoid organs have different requirements for cytokines and growth factors, such as the requirement for IL-7 in Peyer’s patch development and the requirement for TRANCE in lymph node development. However, it remains an open question whether all secondary lymphoid organs have slightly different populations of LTo cells and whether the differences in these populations are intrinsic or are caused by the local environment.
Lymphotoxin signaling and secondary lymphoid organ development
One of the key events in the development of secondary lymphoid organs is lymphotoxin signalling, which promotes the differentiation of mesenchymal LTo cells and leads to the expression of various chemokines and adhesion molecules that are necessary for secondary lymphoid organ development. The essential role of LT is illustrated by Lta−/− mice, which lack Peyer’s patches and most lymph nodes, except for rudimentary mesenteric lymph nodes that occasionally appear in some mice (44, 45). This was initially somewhat surprising, since LTα is a soluble cytokine that is structurally and functionally related to TNF (46). In fact, LTα binds to both TNFR1 and TNFR2, suggesting that the biological functions of LTα and TNF should be very similar (47). However, Tnf−/− mice have normal numbers of lymph nodes and Peyer’s patches, albeit with some structural alterations (48, 49). Moreover, Tnfr1−/− and Tnfr2−/− mice also have normal numbers of secondary lymphoid organs (48, 50), demonstrating that LTα signalling through TNFR1 or TNFR2 is not responsible for secondary lymphoid organ development.
This discrepancy was resolved by the observation that LTα also forms heterotrimers with LTβ, which is expressed as a transmembrane protein (51, 52). The LTαβ heterotrimer is expressed on the cell surface of lymphocytes (53) as well as on LTi and LTin cells (16) and binds to the LTβR. Since the organogenesis of secondary lymphoid organs is disrupted in Lt3−/− mice as well as Ltbr−/− mice (54, 55), it is clear that the surface form of LTαβ must be primarily responsible for triggering secondary lymphoid organ development. However, Ltb−/− mice still retain cervical and mesenteric lymph nodes (54), whereas Lta−/− mice lack these organs (44). Therefore, the soluble form of LTα must cooperate with surface LTαβ to contribute to the development of some lymph nodes. Furthermore, since all lymph nodes and Peyer’s patches are absent from Ltbr−/− mice (55), it is likely that another TNF family member, such as LIGHT (56, 57), acts through LTβR to facilitate the development of cervical and mesenteric lymph nodes.
In fact, TNF, LTα, LTβ and LIGHT all participate in secondary lymphoid organ development to some degree. For instance, mice treated in utero with soluble LTβR fused with the Fc protion of immunoglobulin (LTβR-Ig) retain mesenteric and cervical lymph nodes (58, 59), whereas mice treated in utero with both LTβR-Ig and TNFR1-Ig do not develop these organs (60). Moreover, mice treated with LTβR-Ig and blocking antibodies against TNF also lack all lymph nodes and Peyer’s patches (60), indicating that TNF signaling through TNFR1 plays some role in mesenteric lymph node development. As discussed above, LIGHT also contributes to mesenteric lymph node development. Although both Light−/− and Lt3−/− mice have mesenteric lymph nodes (57), a portion of Light-Lt3−/− mice lack mesenteric lymph nodes (57), suggesting that LIGHT and LTβ cooperate to induce mesenteric lymph node formation. Furthermore, since all Ltbr−/− mice lack mesenteric lymph nodes (55), these results also suggest that there may be another ligand for the LTβR that facilitates mesenteric lymph node development. Thus, although LTαβ signaling through the LTβR is the major pathway through which secondary lymphoid organ development is triggered, other TNF family members also contribute to lymph node development.
Despite the essential nature of interactions between LTαβ-expressing LTi cells and LTβR-expressing LTo cells, this interaction alone is not sufficient to promote secondary lymphoid organ development. For example, although the administration of an agonistic anti-LTβR antibody in utero promotes lymph node development in Lta−/− mice, the administration of this same antibody in Rorc−/− mice, which lack LTi cells, does not promote lymph node development (19). Furthermore, the administration of anti-LTβR to Cxcl13−/− mice also fails to promote lymph node development. These data demonstrate that LTi cells or possibly LTin cells provide additional signals to mesenchymal or stromal cells that are necessary for secondary lymphoid organ development. The identification of these additional signals is key to our future understanding of secondary lymphoid organ development.
Most TNFR family members are coupled to the NFκB signaling pathway (61) and not surprisingly, molecules important in this signalling pathway are involved in LTβR signaling and lymphoid organ development. In particular, triggering of the LTβR induces the canonical pathway of NFκB activation that involves IKKβ and IKKγ/NEMO and leads to the phosphorylation of IκB and the translocation of p50/RelA or p52/RelA complexes to the nucleus (62). In turn, these complexes control the expression of inflammatory proteins, including VCAM-1, MIP1β and MIP2 (62). This pathway also promotes an increased production of the NFκB2/p100 precursor (62). However, LTβR signalling also induces a second pathway that leads to the sequential activation of NIK and IKKα and leads to the processing of p100 to p52 (62, 63). In association with RelB, p52 translocates to the nucleus and activates transcription of molecules involved in secondary lymphoid organ development and homeostasis (62). These molecules include the homeostatic chemokines, CCL19, CCL21 and CXCL13. Both pathways appear to be important in lymphoid organ development, since mice homozygous for a natural mutation in NIK (alymphoplasia or aly/aly mice)(64, 65) as well as Tnfr1-Rela−/− mice lack lymph nodes and Peyer’s patches (66). Furthermore, RelB is essential for Peyer’s patch organogenesis and aspects of splenic architecture (67–69). These results partly explain why LTβR and NIK signaling pathways are essential for lymphoid organ development and also explain why signaling through TNFR1 can facilitate lymph node development under some circumstances.
Lymphotoxin signaling elicits chemokine and adhesion molecule expression by stromal and endothelial cells
As mentioned above, interactions between LTαβ-expressing LTi and LTin cells promote the differentiation of LTβR expressing mesenchymal and endothelial cells (7, 11, 34). As part of this differentiation pathway, the mesenchymal cells express adhesion molecules, such as VCAM-1, ICAM-1 and MAdCAM as well as homeostatic chemokines, including CXCL13, CCL19 and CCL21. LTi cells, and probably LTin cells express chemokine receptors, such as CXCR5 - the receptor for CXCL13, and CCR7 - the receptor for CCL19 and CCL21 (40). LTi cells also express the integrins α4β7 and α4β1 (27, 70), which bind to the vascular adhesion molecule, MAdCAM and VCAM-1 respectively. Homeostatic chemokines, particularly CXCL13, are expressed in Peyer’s patch anlage during development (39). This low level expression attracts CXCR5-bearing IL-7Rα+CD4+CD3− cells to the Peyer’s patch anlage and activates α4β1 integrin in order to facilitate adhesion to the local stroma (20). Once IL-7Rα+CD4+CD3−cells have arrived at the future sites of Peyer’s patch genesis, they provide surface LTαβ (27), which induces the expression of higher levels of chemokines as well as adhesion molecules on the mesenchymal cells (39, 71, 72). These newly expressed molecules then recruit lymphocytes, which provide a sustained source of surface lymphotoxin and induce the differentiation of local stromal cells (73). The chemokine receptors and integrins expressed by LTin cells are currently unknown. However, since LTin cells cluster with LTi cells in the developing Peyer’s patch at the same time, it is probably safe to assume that they express similar chemokine receptors and adhesion molecules.
Homeostatic chemokines do more than simply attract LTi cells to sites of secondary lymphoid organ development. Like IL-7 and TRANCE, they promote the expression of LTαβ on the surface of LTi cells and lymphocytes (40, 41). For example, CXCL13 promotes the expression of LTαβ on the surface of LTi cells as well as on mature B cells (41), whereas CCL21 and CCL19 promote the expression of LTαβ on LTi cells as well as mature CD4 T cells (40). In fact, the expression of homeostatic chemokines and lymphotoxin is co-dependent in a positive feedback loop (41), in which lymphotoxin signalling triggers the expression of homeostatic chemokines by mesenchymal/stromal cells and chemokine signalling maintains expression of LTαβ on the surface of LTi cells and lymphocytes. The essential role of homeostatic chemokines in secondary lymphoid organ development is demonstrated by Cxcl13−/− mice, which fail to develop Peyer’s patches and most lymph nodes (41). Cxcr5−/− mice also lack Peyer’s patches and most lymph nodes (74). Although mice with a natural mutation (paucity of lymph node T cells - plt) that disrupts CCL19 and CCL21 generally form a full complement of secondary lymphoid organs (75–78), the lymph nodes and Peyer’s patches that do develop in plt/plt mice are small and poorly organized (75–77). In part, this is due to the reduced capacity of these organs to recruit mature lymphocytes from the blood and activated antigen presenting cells from regional tissues (75–77, 79–81). However, CCL19 and CCL21 also play a role in the development of secondary lymphoid organs as shown by mice triply deficient in CXCL13, CCL19 and CCL21, which exhibit a more frequent absence of facial and cervical lymph nodes than mice lacking CXCL13 alone (40). The loss of these additional lymph nodes is attributed to poor recruitment of LTi cells as well as the failure of LTi cells to upregulate LTαβ and to promote the differentiation of local mesenchymal cells (40).
Programmed colonization and development of follicular structures in developing lymphoid organs
Although IL-7Rα+CD4+CD3− LTi cells and VCAM+ICAM+ mesenchymal cells are initially arranged in homogenous clusters, they begin to segregate into follicular structures around day E18 (82). CD11c+ cells also segregate into follicular structures in the developing Peyer’s patches at this time (82), although it is not clear whether these cells are CD11c+ LTin cells or whether they are CD11c+ dendritic cells that have differentiated in situ from LTi cells (27, 70). Regardless, the initial follicular formation requires LTαβ and occurs independently of B and T cells (82). Similar events are thought to take place during lymph node development (34). This is step 3 as shown in Figure 1.
The last steps in the development of fully mature secondary lymphoid organs involve the recruitment of lymphocytes, the segregation of B and T cell areas and the formation of mature B cell follicles. In mouse lymph nodes this process is delayed until day 3 after birth (83) due to the sequential expression of MAdCAM and PNAd on HEVs (83, 84). Prior to day 3, MAdCAM-1 is the only vascular addressin expressed on HEVs (83). As a result, cells entering developing lymph nodes during this time must express α4β7, the receptor for MAdCAM. Cells that use this pathway include CD4+CD3− LTi cells and γδT cells (27, 70, 84). However, around day 3, MAdCAM expression is downregulated on the HEVs of lymph nodes and PNAd expression is upregulated, allowing the entry of mature naïve B and T cells (83). This developmental switch is controlled in part by the de novo expression of CD34 and GlyCAM-1 (83), which are substrates for the sulfation reaction that generates the PNAd epitope.
After B and T cells start arriving in the developing lymph node, they begin to take over the role of CD4+CD3− LTi cells by expressing LTαβ and maintaining the differentiation and survival of LTβR-expressing mesenchymal and stromal cells. When B cells first arrive in the developing lymph node, they do not respond to CXCL13 and lack surface LTαβ expression (85). At this time, CXCL13 expression is still dependent on LTαβ expressed by CD4+CD3- LTi cells (85). The lymph node becomes more organized upon the entry of T cells, when B cells begin to segregate in the outer cortex. Interestingly, the initial segregation of T and B cells occurs independently of CXCL13. This is step 4 as shown in Figure 1. However, from day 4 on, the architectural changes are dependent on CXCL13 and CXCL13-responsive B cells. B cells also express LTαβ at this time (85), suggesting that LTαβ-expressing B cells play a major role in maintaining the architecture of secondary lymphoid organs. Consistent with the idea that LTαβ-expressing lymphocytes take over the role of LTαβ-expressing LTi cells, mice that lack NK, B and T cells develop the initial lymph node anlagen, which persists for a while after birth before dissipating (86). Interestingly, the adoptive transfer of IL-7Rα+ T cells or NK cells, but not B cells, restores the final stages of lymph node development (86), suggesting that these cells have the ability to replace LTi cells as the source of LTαβ at this later stage. Although it is not totally understood why adoptively transferred NK and T cells would be more efficient at promoting lymph node maturation than B cells, the authors of this study speculate that IL-7-driven homeostatic expansion of NK and T cells may play a role (86).
Role of LT in the maintenance of lymphoid architecture
Lymphotoxin and TNF expression are also required for the maintenance of lymphoid architecture in adults as well as the appropriate homing and segregation of B and T cells within secondary lymphoid organs (44, 45). For example, the spleens of Lt3−/− mice lack organized B and T cell areas (44, 45), marginal zones (45, 87) and germinal centers (87, 88). Moreover, follicular dendritic cells fail to develop in the absence of lymphotoxin or TNF signaling (73, 89, 90). As in the developmental stages of secondary lymphoid organs, the expression of lymphotoxin and homeostatic chemokines are co-dependent in a positive feedback loop in which lymphotoxin promotes the differentiation and survival of the stromal and dendritic cells that express CXCL13, CCL21 and CCL19 (72), whereas chemokine receptor signalling controls the steady state recruitment and positioning of naïve B and T cells and maintains LTαβ expression on B and T cells(41, 72). Most evidence suggests that the expression of LTαβ and TNF on mature B cells is essential for the maintenance of CXCL13 expression and follicular dendritic cells in the B cell follicle (73, 91). However, mice in which LTβ is genetically deleted specifically in the B cell lineage have normal levels of chemokines, follicular dendritic cells and B cell follicles in the lymph nodes (92, 93). In this case, LTαβ expression by T cells is sufficient to maintain these structures. Together, these data suggest that LTαβ expression on both B and T cells is important for the proper maintenance of lymphoid architecture in adults.
In addition to the maintenance of B cell follicles and the segregation of B and T cells areas, lymphotoxin signalling is also important to maintain dendritic cell numbers via homeostatic proliferation (94) and for the activation and maturation of dendritic cells during immune responses (95). The development of gp38-expressing stromal cells in the spleen is also dependent on lymphotoxin-expressing B cells (96). Furthermore, the continued expression of the vascular addressins, PNAd and MAdCAM, on high endothelial venules is dependent on lymphotoxin signaling (60, 97, 98). Interestingly, soluble LTα and membrane bound LTαβ are responsible for different aspects of lymphoid architecture. For example, the defects in B and T cell separation are less severe in the spleens of Lt3−/− mice(54) than they are in the spleens of Lt3−/− mice (45) and Ltbr−/− mice (55), suggesting that LTα signaling through TNFR1 as well as LTαβ signaling through LTβR is important for lymphocyte organization. In contrast, the presence of dendritic cells in the spleen as well as the expression of MAdCAM and PNAd on high endothelial venules and in marginal zone sinuses is controlled by surface LTαβ and the LTβR but not by TNF, LTα or TNFR1 (59, 94). Finally, the differentiation of follicular dendritic cells requires TNF, LTα and LTβ (49, 73, 90, 99). Thus, various aspects of lymphoid architecture are controlled by the overlapping, yet distinct, activities of soluble LTα, membrane LTαβ and TNF.
Secondary Lymphoid Organogenesis development is strictly confined to a developmental window
The development of conventional secondary lymphoid organs occurs during a temporal window in embryogenesis that varies depending on the particular lymphoid organ (58). For example, mesenteric lymph nodes develop first (around day E9–E10), followed by brachial (day E13), axillary (day E15) inguinal (day E16) and popliteal lymph nodes (day E17)(58). Mucosal lymphoid organs, such as Peyer’s patches and NALT appear to develop last, as disruption of lymphotoxin signaling just before birth blocks Peyer’s patch formation (58) while the adoptive transfer of LTi cells can restore the development of NALT in neonatal Id2−/− mice (21) and the development of Peyer’s patches in neonatal Cxcr5−/− mice (20). In fact, there is a strict developmental window within which the organogenesis of lymph nodes and Peyer’s patches must occur (58). Once this window has passed, the development of these organs cannot take place, regardless of whether lymphotoxin signalling is restored. There also seems to be a developmental window that restricts the formation of lymphoid tissues at ectopic sites, as demonstrated by the intradermal transfer of cells from dissociated lymph nodes to recipient mice (100). When given to adults, the transferred cells formed disorganized clumps. However, when given to neonates, the transferred cells organize into follicular structures with segregated T cell zones and B cell follicles that contain follicular dendritic cells (100). In contrast, there does not seem to be a fixed developmental window for the formation of ILFs. Although ILF development is dependent on lymphotoxin signalling (101, 102), these structures can be restored in adult Lta−/− mice by reconstitution with normal hematopoietic cells (102). Although it is unclear why these particular tissues lack a fixed developmental window, it is known that RORγ-expressing CD4+CD3− LTi-like cells are found in the cryptopatches of adults (10). Thus, the developmental window for each lymphoid organ may be restricted by the availability of LTi cells in that location.
Interestingly, there is even a developmental window that restricts the formation of some splenic structures, such as the gp38-expressing stromal cells in the T cell areas of the spleen (96). The development of these stromal cells requires LTαβ-expressing B cells during the neonatal period (96). As a result, gp38+ stromal cells cannot be restored in the spleens of adult B cell deficient or Lta−/− mice by reconstitution with normal bone marrow (96). On the other hand, most of the other defects in splenic architecture, including the differentiation of follicular dendritic cells, expression of homeostatic chemokines, formation of the marginal zone and the development of germinal centers after immunization, can be reversed upon reconstitution of Lt3−/− mice with normal bone marrow (73, 87, 90, 99).
A role for CD4+CD3- LTi cells after lymphoid organogenesis
One of the most intriguing questions in lymphoid organogenesis is whether the sole purpose of CD4+CD3− LTi cells is to promote secondary lymphoid organ development during a narrow temporal window in fetal and neonatal development or whether these cells are also present and functional in adults (103). Although LTi cells are easily located with relatively high frequency within the early anlagen of developing Peyer’s patches and lymph nodes (14, 34), they are difficult to locate in adults. In fact, the presence of LTi cells in adults remains a controversial point (104). However, RORγ+ LTi-like cells can be observed within the cryptopatches of the adult gut (105), where they are postulated to promote the development of ILFs in response to microbial stimulation (106). CD4+CD3− LTi cells are also proposed to regulate Th2 responses in the spleen (107). CD4+CD3− cells in adult spleens appear to have a program of gene expression similar to that in CD4+CD3− cells found at sites of embryonic lymph node development. For example, CD4+CD3−cells in adults express high levels of LTαβ and TNF. (104). Moreover, the adoptive transfer of normal CD4+CD3− cells from either embryonic or adult tissues into Lt3−/− mice leads to the segregation of B and T cell areas and the expression of CCL21 (108). However, unlike embryonic LTi cells, adult CD4+CD3−cells express high levels of OX40 ligand and CD30 ligand (109). Therefore, the precise lineage relationship between embryonic LTi cells and adult CD4+CD3− cells remains unclear.
To make matters even more complicated, there is evidence that LTi cells in embryo are not a single homogenous population. For example, the development of NALT occurs in the absence of RORγ (110), whereas it is completely dependent on Id2 (21). Since both RORγ and Id2 are thought to be essential for the differentiation of LTi cells (18, 19, 111) and since normal LTi cells restore NALT development in Id2−/− mice (21), these data suggest that there must be some populations of LTi cells that develop independently of RORγ. These different populations of LTi cells may express different patterns of chemokine receptors that preferentially attract them to particular sites of secondary lymphoid organ development. This would fit with the idea that there are also different populations of mesenchymal LTo cells at sites of Peyer’s patch, mesenteric and peripheral lymph node development that express different arrays of chemokines (43). Thus, the CD4+CD3− LTi-like cells found in the spleens and cryptopatches of adults may simply be a few examples of the multiple types of these intriguing cells.
Exceptions to the paradigm of secondary lymphoid organ development
Although Peyer’s patch organogenesis serves as an instructive model for the organogenesis of all lymphoid organs (11), the molecular requirements for the development of Peyer’s patches and other lymphoid organs are not identical. This is illustrated by the differential requirement of lymph nodes and Peyer’s patches for TRANCE and IL-7 (34, 35). Although these two cytokines seem to share a similar purpose in secondary lymphoid organ development (34), their differential expression at sites of Peyer’s patch and lymph node development leads to their differential importance in the development of these organs.
In contrast, the development of some mucosal lymphoid tissues differs much more substantially from that of Peyer’s patches. For example, although NALT and Peyer’s patches share an architectural similarity, the development of these organs is very different. The organogenesis of NALT occurs during the first week or two after birth (21, 112), which is significantly later than that of Peyer’s patches. As in Peyer’s patch development, CD4+CD3− LTi cells are among the first to appear in the developing NALT anlagen (21, 113). However, the development of NALT does not require RORγ-dependent LTi cells (110). On the other hand, NALT development does require Id2-dependent LTi cells (21). In fact, the organogenesis of NALT can be restored in Id2−/− mice by the adoptive transfer of normal LTi cells to neonatal recipients (21). Most importantly, unlike the development of lymph nodes and Peyer’s patches, the development of NALT is not dependent on the lymphotoxin signalling pathway (21, 110). In fact, NALT is present in Lt3−/−, Lt3−/−, Tnf-Lt3−/−, Ltbr−/− and Tnfr1−/− (21, 110). Moreover, NALT develops normally in mice treated in utero with both soluble LTβR and soluble TNFR1 (21), even though these mice do not develop any lymph nodes or Peyer’s patches. These data argue that none of the ligands that bind to either the LTβR or the TNFR1, including the LTα homotrimer, the LTαβ heterotrimer, TNF or LIGHT, are essential for NALT development. Furthermore, mice with mutations in the NFκB signalling pathway, including aly/aly, Nfkb1−/−, Nfkb2−/− and Relb−/− mice, all have NALT to some degree (114). Together, these data argue that the central feature in the paradigm of secondary lymphoid organ development - the interaction between LTαβ-expressing LTi cells and LTβR-expressing mesenchymal LTo cells - is not a part of the NALT developmental program.
Although it is not clear why NALT development requires IL-7R3+CD4+CD3− cells in Id2−/− mice but not in Rorc−/− mice, the difference may reflect the activities of additional cell types that facilitate NALT development. Since Id2−/− mice lack NK cells as well as multiple populations of dendritic cells (18, 115, 116), and have defects in the CD8 T cell lineage (117), these cells may play important, although currently undefined, roles in NALT development in the absence of RORγ-dependent LTi cells. This would be consistent with other data showing that NK cells participate in the stabilization of lymph nodes during development (86). However, if CD4+CD3− cells are a central component in NALT developmental pathway, even in the absence of lymphotoxin signalling, then what signals might these cells provide during NALT organogenesis? It is clear from other studies that additional signals from LTi cells do play a role in lymph node development, since agonistic antibodies to the LTβR do not promote lymph node development in Rorc−/− mice (19). Defining these interactions will be key to our future understanding of secondary lymphoid organ development.
The discrepancy between Rorc−/− and Id2−/− mice in the relative importance of CD4+CD3− cells in NALT development may also reflect diversity in the LTi population - with some LTi cells being formed independently of RORγ. Consistent with this idea, the LTi cells found in NALT express very low levels of CCR7 and CXCR5 relative to their levels on LTi cells from Peyer’s patches (113). Moreover, NALT develops in the absence of CXCL13, CCL19 and CCL21 (113, 118) and normal numbers of LTi cells migrate to the NALT of Cxcl13−/− or plt/plt mice just after birth (113). These data demonstrate that conventional LTi cells are probably not involved in NALT development and further suggest that additional, as yet undefined chemokines attract LTi cells to the NALT anlage. One of these chemokines might be CCL20, which is normally expressed in the dome epithelium of NALT in a lymphotoxin dependent manner (118). If CCL20 is required for NALT formation, possibly by recruiting CCR6-expressing LTi cells, then NALT development might be more analogous to the development of ILFs, which are highly dependent on CCR6 (119).
Despite the fact that the initial stages of NALT development occur independently of the lymphotoxin signalling pathway, the structure of NALT is severely compromised in Lt3−/−, Tnf-Lt3−/− and Lt3r−/− mice (110, 118). In fact, the B and T cells are not segregated into separate areas, follicular dendritic cells and BP3+ stromal cells do not develop, high endothelial venules cannot be found and the normal array of homeostatic chemokines are not expressed in the NALT of Lta−/− mice (110, 118). However, the architectural defects in the NALT of adult Lta−/− mice can be repaired by the restoration of normal lymphotoxin-expressing hematopoietic cells (110). Again, this is dramatically different than other lymphoid organs, which cannot be reconstituted in Lta−/− mice, even after the transfer of normal bone marrow into irradiated recipients (120). Thus, the structural defects in the NALT of Lta−/− mice are primarily due to the failure of lymphotoxin to promote the differentiation of stromal and endothelial cells and the loss of homeostatic chemokine expression.
The development of ILFs is also different than that of Peyer’s patches, despite the similarity of their locations and presumably their functions in the small intestine. For example, the formation of ILFs is clearly dependent on lymphotoxin signalling, since ILFs are absent in Lt3−/− and aly/aly mice (101, 102, 121). However, like NALT, ILFs are completely restored in Lta−/− mice after reconstitution with normal bone marrow (102, 122). Moreover, even though the treatment of mice in utero with LTβR-Ig or anti-IL-7R blocks Peyer’s patch development, it does not prevent the development of ILFs (101, 122). These data suggest that lymphotoxin signalling is important for the maintenance of ILFs after embryonic development rather than for the developmental formation of ILFs. This is probably analogous to role of lymphotoxin in the formation of NALT, in which lymphotoxin is essential for the expression of chemokines and the differentiation of stromal and endothelial cells (118). An alternative explanation is that lymphotoxin is required for ILF development, but that there is no temporal window in development, during which ILF formation must occur.
As it is in NALT, CCL20 is highly expressed in the dome epithelium of ILFs and seems to attract CCR6+ dendritic cells as well as B and T cells to the dome region (119, 123). The central importance of CCR6/CCL20 interactions in the development of ILFs is demonstrated in Ccr6−/− mice, in which ILFs fail to develop properly (119). Like other homeostatic chemokines, CCL20 expression is dependent on LTα (118),j suggesting that the lymphotoxin-induced expression of CCL20 may be the essential step in ILF formation. While this might imply that LTi cells responsible for ILF formation must express CCR6 (124), it reflects instead a requirement for CCR6 on B cells (119). Given that LTαβ expression on B cells and not T cells is also required for the maturation of ILFs (125), one could speculate that there is a positive feedback loop between CCL20 expression in the dome epithelium of ILFs and LTαβ expression on B cells. Interestingly, B cells in ILFs do not have to be antigen specific to mediate their effects. In fact, normal ILFs develop in mice in which all B cells express a transgenic BCR specific for lysozyme (125). Despite the central role of LTαβ+CCR6+ B cells in ILF formation, ILF development is compromised in Rorc−/− mice (106), suggesting that LTi cells are important for ILF formation. However, it is unclear at this time whether LTi cells are attracted to sites of ILF development via CCL20 or other homeostatic chemokines, like CXCL13, which is also highly expressed in mature ILFs.
Another unusual feature of ILF development unlike that of any other secondary lymphoid organ is that it is linked to microbial exposure (102, 125–127). For example, ILF formation is barely detectable in germfree mice (102) and those ILFs that do develop have only small clusters of c-kit+ cells and essentially lack T cell zones or B cell follicles. However, other investigators find that ILFs (also known as Solitary Intestinal Lymphoid Tissue - SILT) are present in normal numbers in germfree mice, but are very small and immature and lack identifiable B cell follicles (126, 127). Importantly, ILFs return to normal upon restoration of intestinal flora (102). These data argue that the location and number of ILFs is determined developmentally and cannot be altered by inflammation or infection. However, the final maturation and organization of ILFs is dependent on microbial stimuli and can even be enhanced by infection with pathogenic bacteria (128). Together, these data demonstrate that the development of ILFs has some characteristics in common with the development of ectopic lymphoid follicles, but other characteristics of secondary lymphoid organs.
Concluding remarks
Our understanding of the programmed development of secondary lymphoid organs has progressed remarkably over the last decade. We now appreciate that interactions between newly emerging populations of LTi and LTin with immature mesenchymal LTo cells are instrumental in this process and that these interactions are orchestrated by a variety of homeostatic chemokines, cytokines and growth factors. In particular, the lymphotoxin signalling pathway is centrally important for the initial differentiation of mesenchymal LTo cells and for the maintenance of architectural elements that make up the scaffolding of secondary lymphoid organs. However, it is now apparent that the developmental pathways governing each secondary lymphoid organ are often subtly and sometimes dramatically different. These differences are reflected in the variety of cytokines and chemokines that are required for the development of each organ and in the various types of LTi cells and LTo cells that are present in each site. Understanding these differences and determining how they impact the ultimate structure and function of each secondary lymphoid organ will be the challenge for the next decade.
Table 1.
Mutations that impair secondary lymphoid organ development.
| Mutation | Signalling pathway | Cells affected | mLNsa | pLNsb | PPsc | NALT | References |
|---|---|---|---|---|---|---|---|
| Lta−/− | LTβR | stroma | ±d | − | − | ±e | (21, 44, 45, 110) |
| Ltb−/− | LTβR | stroma | ± | − | − | ± | (21, 54, 110) |
| Ltbr−/− | LTβR | stroma | − | − | − | ± | (21, 55, 110) |
| Light−/− | LTβR | stroma | + | + | + | NDf | (57) |
| Light/Ltb−/− | LTβR | stroma | − | − | − | ND | (57) |
| Aly/aly | LTβR | stroma | − | − | − | ± | (21, 65, 110) |
| Il7ra−/− | IL-7R | LTi | + | + | − | ± | (15, 21, 34, 110) |
| Il2rg/rag−/− | IL-7R | LTi | + | + | − | − | (86) |
| Jak3−/− | IL-7R | LTi | + | + | − | ND | (15, 34) |
| Trance−/− | TRANCE-R | LTi | − | − | + | + | (35, 37, 110) |
| Rank−/− | TRANCE-R | LTi | − | − | + | ND | (36) |
| Traf6−/− | TRANCE-R | LTi | − | − | + | ND | (38) |
| Rorc−/− | LTi | − | − | − | + | (10, 19, 110) | |
| Id2−/− | LTi | − | − | − | − | (18, 21) | |
| Ikaros−/− | LTi | − | − | − | ND | (23) | |
| Cxcl13−/− | CXCR5 | LTi/B | − | − | − | ± | (40, 41) |
| Cxcr5−/− | CXCR5 | LTi/B | − | − | − | ND | (74) |
| Plt/plt | CCR7 | LTi/B/T | + | + | + | + | (40, 65) |
| Ccr7−/− | CCR7 | LTi/B/T | + | + | + | ND | (129) |
| Cxcl13/Ccl19/21−/− | CXCR5/CCR7 | LTi/B/T | − | − | − | ± | (40) |
| Ret−/− | RET | LTin | ND | ND | − | ND | (16) |
| Graf51/51 | RET | LTin | ND | ND | ± | ND | (16) |
mesenteric lymph nodes
peripheral lymph nodes
Peyer’s patches
small mLNS developed in a few mice
small, disorganized NALT developed
Not determined
Acknowledgments
This work was supported by the Trudeau Institute, NIH grants HL69409 and AI072689 and by the Sandler Program for Asthma Research.
Terms/Definitions
- 1. Homeostatic chemokine
Homeostatic chemokines, including CXCL12, CXCL13, CCL19, CCL21 and CCL20 are constitutively expressed in secondary lymphoid organs and control the steady state recruitment and positioning of lymphocytes and dendritic cells. The expression of these chemokines is often regulated by lymphotoxin
- 2. Lymph node anlagen
The lymph node or Peyer’s patch anlagen is the unstructured collection of hematopoietic and mesenchymal cells that initially forms at sites of secondary lymphoid organ development
- 3. Developmental window
The development of most secondary lymphoid organs occurs during a defined period in embryogenesis known as a developmental window. Certain developmental steps must be completed during this time period or the ability of these organs to develop is permanently disabled
Acronyms
- 1. Lti
Lymphoid Tissue inducer
- 2. Ltin
Lymphoid Tissue initiator
- 3. Lto
Lymphoid Tissue organizer
- 4. NALT
Nasal Associated Lymphoid Tissue
- 5. ILF
Isolated Lymphoid Follicle
Literature cited
- 1.Goodnow CC. Chance encounters and organized rendezvous. Immunol Rev. 1997;156:5–10. doi: 10.1111/j.1600-065x.1997.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 2.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunisawa J, Fukuyama S, Kiyono H. Mucosa-associated lymphoid tissues in the aerodigestive tract: their shared and divergent traits and their importance to the orchestration of the mucosal immune system. Curr Mol Med. 2005;5:557–72. doi: 10.2174/1566524054863924. [DOI] [PubMed] [Google Scholar]
- 4.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–2. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 7.Cupedo T, Kraal G, Mebius RE. The role of CD45+CD4+CD3− cells in lymphoid organ development. Immunol Rev. 2002;189:41–50. doi: 10.1034/j.1600-065x.2002.18905.x. [DOI] [PubMed] [Google Scholar]
- 8.Cupedo T. Regulation of Lymphoid Organogenesis. Vrije Universiteit Amsterdam; Amsterdam: 2003. p. 144. [Google Scholar]
- 9.Cyster JG. Lymphoid organ development and cell migration. Immunol Rev. 2003;195:5–14. doi: 10.1034/j.1600-065x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 10.Eberl G, Littman DR. The role of the nuclear hormone receptor RORγt in the development of lymph nodes and Peyer’s patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 12.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 13.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2007 doi: 10.1016/j.smim.2007.12.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7Rα+CD3− cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11:643–55. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 15.Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor alpha in the formation of Peyer’s patch anlage. Int Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, Barlow A, Pachnis V, Kioussis D. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature. 2007;446:547–51. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- 17.Fukuyama S, Kiyono H. Neuroregulator RET initiates Peyer’s-patch tissue genesis. Immunity. 2007;26:393–5. doi: 10.1016/j.immuni.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Yokoto Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa SI, Gruss P. Development of peripheral lymphoid organs and natual killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–6. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Unutmaz D, Zou Y-R, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORγ in Thymocyte Survival and Lymphoid Organ Development. Science. 2000;288:2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 20.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+CD3- cells induce Peyer’s patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 2002;17:363–73. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 21.Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, Terawaki S, Shikina T, Yamamoto M, Kurono Y, Kiyono H. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3−CD4+CD45+ cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 22.Georgopoulos K, Winandy S, Avitahl N. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997;15:155–76. doi: 10.1146/annurev.immunol.15.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–49. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7:483–92. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 25.Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–54. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Mebius RE, Miyamoto T, Christensen J, Domen J, Cupedo T, Weissman IL, Akashi K. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J Immunol. 2001;166:6593–601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- 27.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells, but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 28.Sabin FR. Further evidence on the origin of lymphatic endothelium from the endothelium of the blood vasculature system. Anat Rec. 1908;2:46–55. [Google Scholar]
- 29.Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic ducts in the pig. Am J Anat. 1902;1:367–81. [Google Scholar]
- 30.Wilting J, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Borges J, Stark GB, Alitalo K, Tomarev SI, Niemeyer C, Rossler J. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. Faseb J. 2002;16:1271–3. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]
- 31.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 32.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, Kraal G, Mebius RE. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173:2968–75. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity. 2002;17:823–33. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, Rennert PD, Choi Y. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–78. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong Y, Yoshida H, Sarosi I, Tan H, Timms E, Capparelli C, Morony S, Olivera-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey D, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 38.Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4:353–62. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 39.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, Nishikawa S-I. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–30. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–8. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansel KM, Ngo VN, Hayman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 42.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 43.Okuda M, Togawa A, Wada H, Nishikawa S. Distinct activities of stromal cells involved in the organogenesis of lymph nodes and Peyer’s patches. J Immunol. 2007;179:804–11. doi: 10.4049/jimmunol.179.2.804. [DOI] [PubMed] [Google Scholar]
- 44.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin α deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–93. [PubMed] [Google Scholar]
- 45.de Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, Russell JH, Karr R, Chaplin DD. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–7. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 46.Banner DW, D’Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W. Crystal Structure of the soluble human 55 kd TNF receptor-human TNFβ complex: Implications for TNF receptor activation. Cell. 1993;73:431–45. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318:665–7. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 48.Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bluethmann H, Kollias G. Peyer’s patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc Natl Acad Sci (USA) 1997;94:6319–23. doi: 10.1073/pnas.94.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFa-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillet N, Sheehan KCF, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumor necrosis factor but normal T cell development in TNF receptor 2 deficient mice. Nature. 1994;372:560–3. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 51.Androlewicz MJ, Browning JL, Ware CF. Lymphotoxin is expressed as a heteromeric complex with a distinct 33-kDa glycoprotein on the surface of an activated human T cell hybridoma. J Biol Chem. 1992;267:2542–7. [PubMed] [Google Scholar]
- 52.Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O’Brine-Greco B, Foley SF, Ware CF. Lymphotoxin b, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–56. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 53.Ware CF, Crowe PD, Grayson MH, Androlewicz MJ, Browning JL. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B and natural killer cells. J Immunol. 1992;149:3881–8. [PubMed] [Google Scholar]
- 54.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenisis for lymphotoxins α and β revealed in lymphotoxin β deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 55.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin b receptor controls organogenisis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Foster A, Chin R, Yu P, Sun Y, Wang Y, Pfeffer K, Fu YX. The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur J Immunol. 2002;32:1969–79. doi: 10.1002/1521-4141(200207)32:7<1969::AID-IMMU1969>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 57.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–24. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rennert PD, Browning JL, Mebius RE, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rennert PD, Browning JL, Hochman PS. Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Int, Immunol. 1997;9:1627–39. doi: 10.1093/intimm/9.11.1627. [DOI] [PubMed] [Google Scholar]
- 60.Rennert PD, James D, Mackay F, Browning JL. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity. 1998;9:71–9. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 61.Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10:693–5. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 62.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–35. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 63.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 64.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuli M, Kogishi K, Serikawa T, Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding NfkB-inducing kinase. Nat Genet. 1999;22:74–7. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 65.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–34. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 66.Alcamo E, Hacohen N, Schulte LC, Rennert PD, Hynes RO, Baltimore D. Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs. J Exp Med. 2002;195:233–44. doi: 10.1084/jem.20011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. Embo J. 2003;22:121–30. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weih DS, Yilmaz ZB, Weih F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol. 2001;167:1909–19. doi: 10.4049/jimmunol.167.4.1909. [DOI] [PubMed] [Google Scholar]
- 69.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–40. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida H, Kawamoto H, Santee SM, Hashi H, Honda K, Nishikawa S, Ware CF, Katsura Y, Nishikawa SI. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167:2511–21. doi: 10.4049/jimmunol.167.5.2511. [DOI] [PubMed] [Google Scholar]
- 71.Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JR, Ruddle NH. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol. 1998;161:6853–60. [PubMed] [Google Scholar]
- 72.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgewick JD, Cyster JG. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–12. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, Rajewski K, Pfeffer K. Mature follicular dendritic cell networks depend on expression of lymphotoxin b receptor by radioresistant stromal cells and of lymphotoxin β and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–67. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A Putative Chemokine Receptor, BLR1, Directs B Cell Migration to Defines Lymphoid Organs and Specific Anatomic Compartments of the Spleen. Cell. 1996;87:1037–47. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 75.Nakano H, Tamura T, Yoshimoto T, Yagita H, Miyasaka M, Butcher EC, Nariuchi H, Kakiuchi T, Matsuzawa A. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur J Immunol. 1997;27:215–21. doi: 10.1002/eji.1830270132. [DOI] [PubMed] [Google Scholar]
- 76.Nakano H, Mori S, Yonekawa H, Nariuchi H, Matsuzawa A, Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–95. [PubMed] [Google Scholar]
- 77.Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol. 2001;166:361–9. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- 78.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone Stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, Hedrick JA, Lira SA. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med. 1999;190:1183–8. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashi H, Yoshida H, Honda K, Fraser S, Kubo H, Awane M, Takabayashi A, Nakano H, Yamaoka Y, Nishikawa S. Compartmentalization of Peyer’s patch anlagen before lymphocyte entry. J Immunol. 2001;166:3702–9. doi: 10.4049/jimmunol.166.6.3702. [DOI] [PubMed] [Google Scholar]
- 83.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3− cells to colonize lymph nodes. Proc Natl Acad Sci U S A. 1996;93:11019–24. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mebius RE, Schadee-Eestermans IL, Weissman IL. MAdCAM-1 dependent colonization of developing lymph nodes involves a unique subset of CD4+CD3− hematolymphoid cells. Cell Adhes Commun. 1998;6:97–103. doi: 10.3109/15419069809004464. [DOI] [PubMed] [Google Scholar]
- 85.Cupedo T, Lund FE, Ngo VN, Randall TD, Jansen W, Greuter MJ, de Waal-Malefyt R, Kraal G, Cyster JG, Mebius RE. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J Immunol. 2004;173:4889–96. doi: 10.4049/jimmunol.173.8.4889. [DOI] [PubMed] [Google Scholar]
- 86.Coles MC, Veiga-Fernandes H, Foster KE, Norton T, Pagakis SN, Seddon B, Kioussis D. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc Natl Acad Sci U S A. 2006;103:13457–62. doi: 10.1073/pnas.0604183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type 1 TNF receptor in the formation of germinal centers. Science. 1996;271:1289–91. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 88.Matsumoto M, Lo SF, Carruthers CJL, Min J, Mariathasan S, Huang G, Plas DR, Martin SM, Geha RS, Nahm MH, Chaplin DD. Affinity maturation without germinal centers in lymphotoxin a deficient mice. Nature. 1996;382:462–6. doi: 10.1038/382462a0. [DOI] [PubMed] [Google Scholar]
- 89.Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin a dependent fashion. J Exp Med. 1998;187:1009–18. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hir ML, Bluethmann H, Kosco-Vilbois MH, Muller M, di Padova F, Moore M, Ryffel B, Eugster H-P. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor 1 signaling. J Exp Med. 1996;183:2367–72. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu Y-X, Huang G, Matsumoto M, Molina H, Chaplin DD. Independent signals regulate development of primary and secondary follicular structure in spleen and mesenteric lymph node. Proc Natl Acad Sci. 1997;94:5739–43. doi: 10.1073/pnas.94.11.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tumanov AV, Kuprash DV, Mach JA, Nedospasov SA, Chervonsky AV. Lymphotoxin and TNF produced by B cells are dispensable for maintenance of the follicle-associated epithelium but are required for development of lymphoid follicles in the Peyer’s patches. J Immunol. 2004;173:86–91. doi: 10.4049/jimmunol.173.1.86. [DOI] [PubMed] [Google Scholar]
- 93.Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–50. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 94.Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu X-Y. The requirement on membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J Exp Med. 1999;190:629–38. doi: 10.1084/jem.190.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Summers-DeLuca LE, McCarthy DD, Cosovic B, Ward LA, Lo CC, Scheu S, Pfeffer K, Gommerman JL. Expression of lymphotoxin-alphabeta on antigen-specific T cells is required for DC function. J Exp Med. 2007;204:1071–81. doi: 10.1084/jem.20061968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–60. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eugster H-P, Muller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, hir ML, di Padova F, Aguet M, Zinkernagel R, Bluethmann H, Ryffel B. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-a double deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 98.Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–50. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 99.Fu Y-X, Molina H, Matsumoto M, Huang G, min J, Chaplin DD. Lymphotoxin a (LTa) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–20. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21:655–67. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 101.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 102.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–82. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 103.Lane PJ, Gaspal FM, Kim MY. Two sides of a cellular coin: CD4(+)CD3- cells regulate memory responses and lymph-node organization. Nat Rev Immunol. 2005;5:655–60. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim MY, Toellner KM, White A, McConnell FM, Gaspal FM, Parnell SM, Jenkinson E, Anderson G, Lane PJ. Neonatal and adult CD4+ CD3- cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15) J Immunol. 2006;177:3074–81. doi: 10.4049/jimmunol.177.5.3074. [DOI] [PubMed] [Google Scholar]
- 105.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–51. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 106.Ivanov II, Diehl GE, Littman DR. Lymphoid tissue inducer cells in intestinal immunity. Curr Top Microbiol Immunol. 2006;308:59–82. doi: 10.1007/3-540-30657-9_3. [DOI] [PubMed] [Google Scholar]
- 107.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–54. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 108.Kim MY, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V, Walker LS, Caamano J, Jenkinson E, Anderson G, Lane PJ. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–10. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 109.Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174:6686–91. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 110.Harmsen A, Kusser K, Hartson L, Tighe M, Sunshine MJ, Sedgwick JD, Choi Y, Littman DR, Randall TD. Organogenesis of Nasal Associated Lymphoid Tissue (NALT) occurs independently of lymphotoxin-α (LTα) and retinoic acid receptor-related orphan receptor-γ, but the organization of NALT is LTα-dependent. J Immunol. 2002;168:986–90. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- 111.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 112.Ying X, Chan K, Shenoy P, Hill M, Ruddle NH. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am J Pathol. 2005;166:135–46. doi: 10.1016/S0002-9440(10)62239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fukuyama S, Nagatake T, Kim DY, Takamura K, Park EJ, Kaisho T, Tanaka N, Kurono Y, Kiyono H. Cutting edge: Uniqueness of lymphoid chemokine requirement for the initiation and maturation of nasopharynx-associated lymphoid tissue organogenesis. J Immunol. 2006;177:4276–80. doi: 10.4049/jimmunol.177.7.4276. [DOI] [PubMed] [Google Scholar]
- 114.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 115.Ikawa T, Fujimoto S, Kawamoto H, Katsura Y, Yokota Y. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci U S A. 2001;98:5164–9. doi: 10.1073/pnas.091537598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, Zenke M. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 117.Kusunoki T, Sugai M, Katakai T, Omatsu Y, Iyoda T, Inaba K, Nakahata T, Shimizu A, Yokota Y. TH2 dominance and defective development of a CD8+ dendritic cell subset in Id2-deficient mice. J Allergy Clin Immunol. 2003;111:136–42. doi: 10.1067/mai.2003.29. [DOI] [PubMed] [Google Scholar]
- 118.Rangel-Moreno J, Moyron-Quiroz J, Kusser K, Hartson L, Nakano H, Randall TD. Role of CXC Chemokine Ligand 13, CC Chemokine Ligand (CCL) 19, and CCL21 in the Organization and Function of Nasal-Associated Lymphoid Tissue. J Immunol. 2005;175:4904–13. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- 119.McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–40. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 121.Taylor RT, Patel SR, Lin E, Butler BR, Lake JG, Newberry RD, Williams IR. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol. 2007;178:5659–67. doi: 10.4049/jimmunol.178.9.5659. [DOI] [PubMed] [Google Scholar]
- 122.Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, Hiroi T, Nanno M, Kiyono H. Prenatal blockage of lymphotoxin beta receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174:4365–72. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- 123.Wang C, McDonald KG, McDonough JS, Newberry RD. Murine isolated lymphoid follicles contain follicular B lymphocytes with a mucosal phenotype. Am J Physiol Gastrointest Liver Physiol. 2006;291:G595–604. doi: 10.1152/ajpgi.00525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lugering A, Kucharzik T. Induction of intestinal lymphoid tissue: the role of cryptopatches. Ann N Y Acad Sci. 2006;1072:210–7. doi: 10.1196/annals.1326.015. [DOI] [PubMed] [Google Scholar]
- 125.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–8. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 126.Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, Worbs T, Macpherson AJ, Forster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177:6824–32. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- 127.Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 128.Halle S, Bumann D, Herbrand H, Willer Y, Dahne S, Forster R, Pabst O. Solitary intestinal lymphoid tissue provides a productive port of entry for Salmonella enterica serovar Typhimurium. Infect Immun. 2007;75:1577–85. doi: 10.1128/IAI.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 Coordinates the Primary Immune Response by Establishing Functional Microenvironments in Secondary Lymphoid Organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]