Abstract
Arf GTPases control vesicle formation from different intracellular membranes and are regulated by Arf guanine nucleotide exchange factors (GEFs). Outside of their conserved catalytic domains, known as Sec7 domains, little is known about Arf GEFs. Rsp5 is a yeast ubiquitin ligase that regulates numerous membrane trafficking events and carries a C2 domain that is specifically required for trans-Golgi network to vacuole transport. In a screen for proteins that interact with the Rsp5 C2 domain we identified Sec7, the GEF that acts on Golgi-associated Arfs. The Rsp5-Sec7 interaction is direct, occurs in vivo, and is conserved among mammalian Rsp5 and Sec7 homologues. A 50-amino acid region near the Sec7 C terminus is required for Rsp5 binding and for normal Sec7 localization. Binding of Sec7 to Rsp5 is dependent on the presence of the phosphoinositide 3-kinase Vps34, suggesting that phosphatidylinositol 3-phosphate (PI(3)P) plays a role in regulating this interaction. Overexpression of Sec7 significantly suppresses the growth and sorting defects of an rsp5 C2 domain point mutant. These observations identify a new functional region within the Sec7/BIG family of Arf GEFs that is required for trans-Golgi network localization.
Newly synthesized proteins are transported from the Golgi to either the plasma membrane or the lysosome. This sorting decision is made at the trans-Golgi network (TGN)5 where separation of secreted proteins from lysosomal proteins occurs. In mammalian cells sorting at the TGN targets proteins for secretion, to apical or basolateral membranes, or to the lysosome. In yeast, proteins follow similar pathways, although the distinction between plasma membrane compartments does not exist and the intracellular destination is the lysosome-like vacuole.
Many proteins involved in these trafficking pathways have been identified and characterized in yeast and are conserved in mammalian cells (reviewed in Refs. 1–3). Some integral membrane cargos destined for the lysosome are modified with a monoubiquitin signal at the TGN by members of the Nedd4/Rsp5 family of ubiquitin ligases (reviewed in Refs. 4 and 5). Other integral membrane and luminal proteins do not require modification with ubiquitin for efficient trafficking from the TGN to the endosomal sorting pathway. However, the role of ubiquitin in sorting of these cargos within the endosomal pathway remains unclear (6–10). Ubiquitinated cargo is recognized at the Golgi by the ubiquitin-binding domains in GGA (Golgi localized, γ-ear containing, Arf-binding) proteins that sort TGN cargo into vesicles (reviewed in Ref. 3). After leaving the TGN, cargo travels to the lysosome through a late endosomal compartment, known as the multivesicular endosome (MVE), where monoubiquitinated cargo is sorted into vesicles that bud into the MVE lumen. The MVE vesicles and their contents are then delivered in their entirety into the lumen of the lysosome (reviewed in Refs. 11 and 12).
Arf proteins are the small GTPases that regulate vesicle formation at the TGN (13, 14). Like other GTPases, Arf proteins are activated upon exchange of bound GDP for GTP in a reaction catalyzed by guanine nucleotide exchange factors (GEFs). Arf activation by GEFs is a crucial regulatory step, because Arf must be in the GTP-bound state to recruit vesicle coat proteins to initiate vesicle budding (15, 16). Arf GEFs are characterized by having a catalytic Sec7 domain, first identified in the Saccharomyces cerevisiae Sec7 protein (reviewed in Refs. 17 and 18).
Sec7 is a large, essential, Golgi-associated Arf GEF required for the formation of transport vesicles from the TGN in yeast (19). It is localized primarily to TGN membranes (20). Mutations in Sec7 cause defects in the transport of a variety of cargo proteins from the Golgi to the vacuole, including proteins that travel by ubiquitin-independent and -dependent pathways (21, 22). Sec7 has two mammalian homologues, BIG1 and BIG2, that primarily localize to the TGN to function in the release of vesicles from this organelle (reviewed in Ref. 23). BIG2 also localizes to the recycling endosome and is involved in organelle integrity (24). Beyond the central catalytic domain, there are no characterized domains within Sec7, and little is known about the interactions and functions of the N- and C-terminal regions of the protein (21). Recently, regions of homology have been identified within BIG1 and BIG2 (reviewed in Ref. 18). These regions are sites of dimerization between BIG proteins and also interact with other binding partners (25–29). However, the function of these interactions is still unclear.
Ubiquitin signals are attached to biosynthetic cargo traveling from the TGN to the lysosome by ubiquitin ligases, regulatory proteins that catalyze the transfer of ubiquitin to substrates (reviewed in Refs. 30 and 31). One family of ubiquitin ligases that plays important roles in a variety of membrane trafficking processes is named for the mammalian Nedd4 and yeast Rsp5 proteins. Rsp5 is the sole S. cerevisiae member of the Nedd4/Rsp5 family; mammalian members include Nedd4–1, Nedd4–2, WWP1/AIP5, WWP2/AIP2, AIP4/Itch, Smurf1, and Smurf2. These ligases have a conserved domain structure, an N-terminal C2 domain, two to four central WW domains, and a C-terminal HECT (homologous to E6AP C terminus) catalytic domain (5, 30, 32).
The function of the C2 domains in these ligases is ambiguous. C2 domains are found in proteins involved in vesicle trafficking, lipid modification, GTPase regulation, and protein phosphorylation (33–35). They bind to both phospholipids and proteins, and binding of Ca2+ to C2 domains can regulate these interactions (34, 36). The C2 domains of Nedd4 and Smurf2 are important for interaction of these proteins with the plasma membrane (37–41). The Smurf2 C2 domain also plays an autoinhibitory function through binding to the HECT domain that inhibits autoubiquitination and substrate ubiquitination (42). The Rsp5 C2 domain regulates trafficking in the late endocytic pathway at the MVE (43–45).
Previously, we demonstrated that the Rsp5 C2 domain binds phosphoinositides (43), particularly PI(3)P, which is enriched on endosomal membranes (reviewed in Ref. 46). Specific amino acids within the Rsp5 C2 domain that are required for PI(3)P binding are also required for the ubiquitination of cargo traveling from the TGN to the MVE, and thus for the sorting of this cargo into MVE vesicles (43). To further understand the function of the Rsp5 C2 domain in TGN to vacuole trafficking, we screened for proteins that bind to and function with the C2 domain. Here we identify the Arf GEF Sec7 as an Rsp5 C2 domain-binding protein in vitro and in vivo, and we investigate the physical and functional characteristics of this interaction.
EXPERIMENTAL PROCEDURES
Strains, Media, and Reagents—Strains used in this study are listed in Table 1. Yeast strains were propagated in rich medium (2% Bacto-peptone, 1% yeast extract, 2% glucose supplemented with 20 mg/liter adenine, uracil, and tryptophan) or selective minimal medium (47). Caffeine-containing medium was prepared by adding caffeine to a final concentration of 6 mm before autoclaving.
TABLE 1.
Yeast strains
| Strain | Genotypea |
|---|---|
| PJ69-4A | trp1–901 leu2–3,112 ura3–52 his3–200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ |
| PJ69-4α | MATα, same as PJ69-4A |
| LHY1107 | pNotI-RSP5[TRP1] rsp5Δ::HIS3 his3 trp1 lys2 ura3 leu2 bar1 |
| LHY1850 | ura3Δ0 leu2Δ0 his3Δ1 met15Δ0 |
| LHY3876 | prsp5K44,45,75,77,78Q[TRP1] pGFP-CPS rsp5Δ::HIS3 his3 leu2 ura3 trp1 bar1 |
| LHY3923 | prsp5K44,45,75,77,78Q[TRP1] rsp5Δ::HIS3 his3 leu2 ura3 trp1 bar1 |
| LHY4007 | prps5K75,77,78Q[TRP1] his3 trp1 lys2 ura3 leu2 bar1 |
| LHY4377 | pRSP5[TRP1] rsp5Δ::HIS3 leu2 ura3 trp1 bar1 |
| LHY4488 | pGFP-C2[TRP1] his3 trp1 lys2 ura3 leu2 bar1 |
| LHY5440 | SEC7-GFP::URA3 ura3Δ0 leu2Δ0 his3Δ1 met15Δ0 |
| LHY5466 | pNotI-RSP5[TRP1] rsp5Δ::HIS3 SEC7-GFP::URA3 his3 trp1 lys2 ura3 leu2 bar1 |
| LHY5467 | pNotI-rsp5ΔC2[TRP1] rsp5Δ::HIS3 SEC7-GFP::URA3 his3 trp1 lys2 ura3 leu2 bar1/bar1::HIS3 |
| LHY5474 | prsp5K44,45,75,77,78Q[TRP1] pSEC7[URA3] rsp5Δ::HIS3 his3 leu2 ura3 trp1 bar1 |
| LHY5476 | prsp5K44,45,75,77,78Q[TRP1] rsp5Δ::HIS3 his3 leu2 ura3 trp1 bar1 |
| LHY5508 | vps34Δ::kanMX4 SEC7-GFP::URA3 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 |
| LHY5512 | prsp5K44,45,75,77,78Q[TRP1] pSEC7[LEU2] pGFP-CPS[URA3] rsp5Δ::HIS3 his3 leu2 ura3 trp1 bar1 |
| LHY5518 | pNotI-RSP5[TRP1] rsp5Δ::HIS3 sec7ΔCIR-GFP::URA3 his3 trp1 lys2 ura3 leu2 bar1 |
| LHY5519 | pNotI-rsp5ΔC2[TRP1] rsp5Δ::HIS3 sec7ΔCIR-GFP::URA3 his3 trp1 lys2 ura3 leu2 bar1/bar1::HIS3 |
| LHY5521 | pRSP5[TRP1] pGFP-CPS[URA3] rsp5Δ::HIS3 leu2 ura3 trp1 bar1 |
| LHY5604 | pNotI-RSP5[TRP1] rsp5Δ::HIS3 Sec7::URA3 SYS1-GFP::LEU2 his3 trp1 lys2 ura3 leu2 bar1 |
| LHY5605 | pNotI-RSP5[TRP1] rsp5Δ::HIS3 sec7ΔCIR::URA3 SYS1-GFP::LEU2 his3 trp1 lys2 ura3 leu2 bar1 |
All strains are MATa unless otherwise noted
Anti-green fluorescent protein (GFP) antibodies were purchased from Roche Applied Sciences and anti-glutathione S-transferase (GST) antibodies were purchased from Amersham Biosciences. Anti-Rsp5 antiserum was previously described (43, 48).
Plasmids—A multicopy SEC7 plasmid (YepTA65) (19, 49) was provided by N. Segev (University of Illinois, Chicago, IL). Plasmids encoding Sys1-GFP and Gap1-GFP were provided by B. Glick (University of Chicago, Chicago, IL) and R. Piper (University of Iowa, Iowa City, IA), respectively. URA3-marked or LEU2-marked plasmids encoding GFP-carboxypeptidase S (CPS) were provided by S. Emr (University of California, San Diego, CA) or generated in our laboratory.6 All site-directed mutagenesis was performed with a QuikChange® mutagenesis kit (Stratagene, La Jolla, CA).
The yeast two-hybrid C2 domain bait vector (LHP2103) was constructed by PCR amplification of DNA encoding aa 1–142 of Rsp5, followed by insertion of the amplified fragment into the yeast two-hybrid GAL4-binding domain vector, pAS2-1 (Clontech, Palo Alto, CA). The 3K→Q (K75Q, K77Q, K78Q) mutation was introduced into the bait plasmid by site-directed mutagenesis (LHP2169).
DNA encoding aa 1836–2009 of Sec7 was amplified from yeast genomic DNA and inserted into the pET-30 bacterial expression vector by ligation-independent cloning (Novagen, Madison, WI). Truncations and deletions of this fragment were introduced by site-directed mutagenesis. The same method was used to construct plasmids encoding the C termini of BIG1 (aa 1665–1849, LHP2396), BIG2 (aa 1567–1785, LHP2378), and Gea1 (aa 1352–1408, LHP2353). Bacterial expression plasmids encoding GST-C2, GST-Sla1350–420, and GST-Rvs167SH3 have been described (43, 50). The 3K→Q mutation was generated in GST-C2 (LHP1665) by site-directed mutagenesis. DNA encoding the Itch C2 domain (aa 6–146, LHP2699) was amplified with terminal BglII sites and ligated into BamHI-digested pGEX-6P-2 (GE Healthcare).
To construct an integrating plasmid for insertion of a C-terminal GFP tag into chromosomal SEC7, we amplified a fragment encoding Sec7 aa 1513–2009 from genomic DNA. This fragment was ligated into pUSE-URA3 (51), a plasmid encoding aa 1815–2009 of Sec7 fused to a GFP, provided by B. Glick (University of Chicago, Chicago, IL). The endogenous SpeI site was removed from the resulting plasmid and another SpeI site was introduced with silent mutations at the codons for aa 1722 and 1723 (LHP2505). An integrating plasmid to construct a chromosomally encoded GFP-tagged Sec7 lacking the C2 domain-interacting region, GFP-Sec7ΔCIR, was similarly made with the additional removal of DNA encoding aa 1836–1883 (LHP2507).
To provide wild-type and sec7 mutant strains for the localization of Sys1-GFP, we constructed untagged SEC7 and sec7ΔCIR integrants. DNA encoding the GFP tag from LHP2505 and LHP2507 was removed by digestion with BamHI and EagI. The ends of the remaining large fragments of the plasmids were converted to blunt ends with T4 DNA Polymerase (New England Biolabs, Beverly, MA) and the plasmids were ligated. A double stop codon was introduced after the codon for amino acid 2009 by site-directed mutagenesis resulting in plasmids encoding DNA to integrate untagged SEC7 (LHP2644) and untagged sec7ΔCIR (LHP2645). Plasmids were digested with SpeI and transformed into yeast. Replacement of the endogenous copy of SEC7 by homologous recombination was verified by PCR amplification of genomic DNA recovered from transformants. All mutations and plasmid sequences were verified by digestion and/or automated sequencing.
Yeast Two-hybrid Screen—A plasmid encoding the binding domain of Gal4 fused to the Rsp5 C2 domain (LHP2103) was used to screen a yeast two-hybrid plasmid library (52). The yeast strain PJ69-4α was transformed with the yeast two-hybrid library and the strain PJ69-4A was transformed with LHP2103. The resulting transformants were mated and diploids were selected and transferred to minimal medium lacking leucine, tryptophan, and adenine to select colonies exhibiting a positive interaction. Plasmids from these colonies were recovered and co-transformed with the original bait plasmid (LHP2103) and a bait plasmid carrying the 3K→Q mutations (LHP2169). Plasmids from colonies that exhibited a positive interaction with the wild-type bait plasmid a second time were sequenced.
Recombinant Protein Purification and Binding Experiments— Hexahistidine (His6)-tagged and GST-tagged recombinant proteins were expressed in BL21-Codon Plus Escherichia coli (Stratagene) propagated in Luria broth supplemented with 40 mg/ml kanamycin, or with 100 mg/ml ampicillin and 20 mg/ml chloramphenicol, for plasmid maintenance. Recombinant protein expression was induced at 18 or 24 °C with 1 mm isopropyl β-d-thiogalactopyranoside (Sigma). Purification of His6-tagged proteins, purification of GST-tagged proteins, and binding experiments with these proteins were performed as previously described (43, 50, 53). Lysates and bound proteins were resolved by SDS-PAGE and analyzed by Coomassie staining or immunoblotting with anti-GST as previously described (43, 48). For binding experiments with GFP-tagged C2 domain (GFP-C2) in yeast lysates, cells were harvested at a density of 1–2 × 107 cells/ml. Lysates were prepared by mechanical agitation with glass beads in MES buffer (1% Triton X-100, 100 mm MES, pH 6.5, 0.5 mm MgCl2, 1 mm EGTA, 0.2 mm dithiothreitol) containing protease inhibitor mixture (0.2 μg/ml chymostatin, 1 μg/ml leupeptin, 2.5 μg/ml antipain, 1 μg/ml pepstatin, 1 mm phenylmethanesulfonyl fluoride). Lysates were incubated on ice for 1 h with 2 mg/ml β-d-maltoside and 1% Triton X-100, cleared by centrifugation, and incubated with immobilized proteins as described for bacterial lysates. Lysates and bound proteins were analyzed by immunoblotting with anti-GFP as previously described (43, 48).
Native Co-immunoprecipitation Experiments—Cells (1.5 × 109) were harvested at a density of 1–2 × 107 cells/ml. Lysates for immunoprecipitation were prepared by mechanical agitation with glass beads in GFP IP buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate) containing protease inhibitor mixture, 2 mg/ml β-d-maltoside, and 1% Triton X-100. Lysates were incubated on ice for 1 h and cleared by centrifugation. Immunoprecipitations were performed overnight at 4 °C with GFP antibodies and Protein G-Sepharose (Amersham Biosciences). Precipitated proteins were washed with GFP IP buffer and GFP IP wash buffer (50 mm Tris-HCl, pH 7.5, 0.25 m NaCl, 0.1% Nonidet P-40, 0.05% deoxycholate), resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Sec7-GFP) or nitrocellulose membranes (Rsp5), and analyzed by immunoblotting as previously described using anti-Rsp5 or anti-GFP (43, 48).
Fluorescence Microscopy—Cells were grown to a density of 1–2 × 107 cells/ml and harvested at 4 °C. Cells were embedded in 1.67% low melt agarose (American Biorganics Inc., Niagra Falls, NY) on a slide and analyzed by fluorescence microscopy (Leica DMIRE2 or Zeiss Axiovert 200M). For quantification of GFP-CPS sorting, cells with a detectable GFP signal were counted and binned into one of three groups based on the location of the GFP signal: group 1, vacuolar lumen (solid spot); group 2, limiting membrane of the vacuole (ring around the vacuole); or group 3, vacuolar lumen and limiting membrane (ring around the vacuole with diffuse internal fluorescence). For Sec7-GFP and Sys1-GFP images, cross-sections were taken along the z axis and projections were made using ImageJ version 1.34.
Growth Suppression Analysis—Cells were transformed with an URA3-marked multicopy plasmid encoding SEC7 (YepTA65, Refs. 19 and 49). To remove the plasmid, cells were grown on medium containing 5-fluoroorotic acid. For serial dilution growth assays, multiple transformants were grown overnight to stationary phase. Cells were serially diluted to 2 × 106, 2 × 105, 2 × 104, or 2 × 103 cells/ml and transferred in duplicate with an inoculating manifold to rich medium with or without 6 mm caffeine. Cells were grown at 37 °C.
RESULTS
The Rsp5 C2 Domain Binds to the Sec7 C Terminus—C2 domains bind to both lipids and proteins (38, 41, 42, 54–57). The Rsp5 C2 domain binds to PI(3)P, however, replacement of the C2 domain with another PI(3)P binding domain, the Fab1 FYVE (Fab1, YOTB, Vac1, and EEA1) domain (58) did not rescue the growth or MVE sorting phenotypes conferred by deletion of the C2 domain of Rsp5.7 One explanation for this observation is that the Rsp5 C2 domain might participate in other interactions important for Rsp5 function.
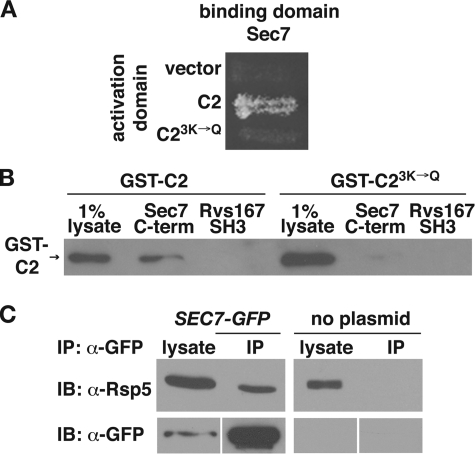
To identify C2 domain-binding proteins, we performed a yeast two-hybrid screen with a bait plasmid encoding the Rsp5 C2 domain. Four clones were identified that encoded the C terminus of the Arf GEF Sec7, spanning amino acids 1836–2009 (Fig. 1A). Mutation of three lysine residues in the Rsp5 C2 domain (K75Q, K77Q, and K78Q, referred to as 3K→Q) inhibits both PI binding and the ubiquitination of cargo destined for entry into MVE vesicles, similar to the effects of the previously published 5K→Q mutant (43). To determine whether K75Q, K77Q, and K78Q are involved in binding to the C terminus of Sec7, we introduced the 3K→Q mutations into the yeast two-hybrid C2 domain bait plasmid (C23K→Q). The Sec7–C2 domain two-hybrid interaction was abolished by these mutations (Fig. 1A), suggesting that the C2 domain Lys75, Lys77, and Lys78 residues are involved in mediating protein-protein interactions as well as protein-lipid interactions.
FIGURE 1.
The C2 domain of Rsp5 binds to the C terminus of Sec7. A, yeast two-hybrid analysis of the interaction between the C terminus of Sec7 (aa 1836–2009) and the wild-type or 3K→Q mutant C2 domain. Yeast strains carrying the indicated activation and binding domain plasmids were incubated on minimal medium at 30 °C. B, lysates prepared from bacteria expressing GST-C2 or GST-C23K→Q were incubated with immobilized His6-Sec71836-2009. Bound proteins were eluted by boiling, resolved by SDS-PAGE, and analyzed by immunoblotting with GST antibodies. His6-Rvs167SH3 was used as a control for nonspecific binding. The presence of equal amounts of immobilized protein in each reaction was verified by Coomassie staining of the eluted, resolved proteins (not shown). C, proteins from yeast cell lysates containing Sec7-GFP (LHY5440) and untagged Sec7 (LHY1850) were immunoprecipitated using GFP antibodies. Total lysates (0.7%, anti-GFP blot and 0.07%, anti-Rsp5 blot) and precipitated proteins were resolved by SDS-PAGE and analyzed by immunoblotting (IB) with GFP or Rsp5 antibodies. IP, immunoprecipitates.
To test whether the C2 domain binds directly to Sec7, a His6-tagged version of the Sec7 C terminus (aa 1836–2009) was expressed in bacteria and immobilized on beads. Bacterial lysates containing GST-C2 or GST-C23K→Q were incubated with the immobilized proteins. GST-C2, but not GST-C23K→Q, bound to the immobilized Sec7 C terminus (Fig. 1B). Neither C2 domain bound to a negative control, the SH3 domain of the endocytic protein Rvs167.
To determine whether Rsp5 and Sec7 interact in the cell, we performed native immunoprecipitations of Sec7-GFP from yeast lysates with anti-GFP. The immunoprecipitates were then analyzed on immunoblots probed with Rsp5 antiserum. Rsp5 was precipitated specifically from lysates containing Sec7-GFP and not from lysates containing untagged Sec7, indicating that Sec7 and Rsp5 interact in vivo (Fig. 1C). Taken together, our observations indicate that Rsp5 binds to Sec7 in vitro and in vivo.
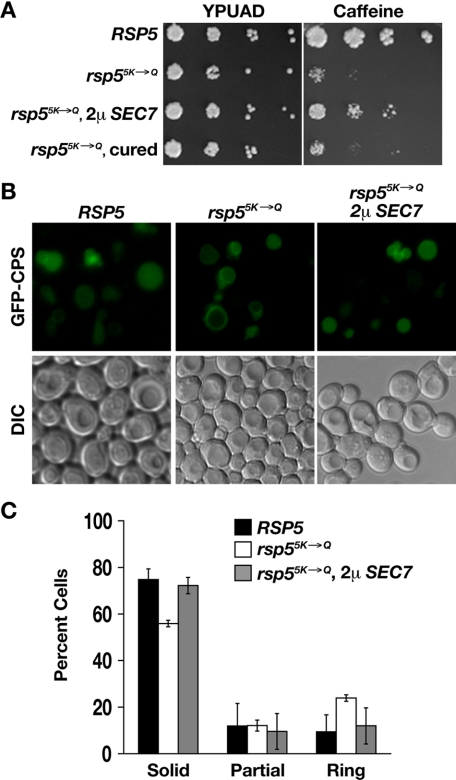
Overexpression of Sec7 Suppresses Phenotypes Conferred by C2 Domain Point Mutations—Both the rsp53K→Q mutant and a C2 domain mutation in which two additional lysines are mutated, rsp55K→Q (K44Q, K45Q, K75Q, K77Q, and K78Q), are sensitive to growth on caffeine-containing medium and are defective in sorting MVE cargo (data not shown, Ref. 43). The growth phenotype of the rsp55K→Q mutant is more severe than that of the rsp53K→Q mutant, resulting in less background growth, therefore we used the rsp55K→Q mutant for the following suppression analysis. To test for a functional relationship between Sec7 and Rsp5, we introduced a multicopy plasmid encoding Sec7 into rsp55K→Q cells. Cells expressing rsp55K→Q as the sole source of Rsp5 grew poorly at 37 °C on medium containing caffeine (Fig. 2A). However, when Sec7 was overexpressed in these cells, growth on caffeine-containing medium improved significantly. Rescue of caffeine-sensitive growth was dependent on the presence of the SEC7 plasmid, because after the plasmid was cured from the cells (see “Experimental Procedures”), the cells reverted to poor growth on caffeine-containing medium (Fig. 2A). Because the requirement for Rsp5, and specifically the C2 domain, can be overcome by overexpression of Sec7, this suggests that these proteins are functioning in the same pathway.
FIGURE 2.
Overexpression of Sec7 suppresses rsp55K→Q phenotypes. A, strains expressing Rsp5 (LHY4377) or Rsp55K→Q (vector, LHY3923; SEC7 plasmid, LHY5474; cured of SEC7 plasmid, LHY5476) were serially diluted and grown at 37 °C on rich medium with or without 6 mm caffeine. All transformants tested exhibited this suppression phenotype. B, localization of GFP-CPS in RSP5 (LHY5521) and rsp55K→Q cells, with or without a multicopy SEC7 plasmid (LHY5512, LHY3876), was visualized by fluorescence and differential interference contrast (DIC) microscopy. C, quantification of the GFP-CPS localization experiment represented in B. In three independent experiments, fluorescent cells were counted (n1 = 868, n2 = 1652, n3 = 3803) and classified by location of the GFP signal: vacuolar lumen (solid), vacuolar limiting membrane (ring), or both the vacuolar lumen and limiting membrane (partial).
GFP-CPS is a cargo protein that is modified with a ubiquitin signal and is transported to the lumen of the vacuole through internalization into luminal MVE vesicles. When GFP-CPS is properly sorted, a fluorescent spot that coincides with the vacuole is observed by fluorescence microscopy. When sorting into MVE vesicles is disrupted, GFP-CPS is observed on the limiting membrane of the vacuole (59). We expressed GFP-CPS in rsp55K→Q cells with or without the SEC7 overexpression plasmid. The rsp5K→Q strain was defective in sorting GFP-CPS to the vacuole lumen, as previously observed (43). Overexpression of Sec7 partially rescued this defect; the cells carrying the SEC7 plasmid showed less staining of the vacuole limiting membrane and more luminal staining (Fig. 2, B and C). These observations suggest a functional relationship between Sec7 and the Rsp5 C2 domain that promotes sorting of cargo into MVE vesicles.
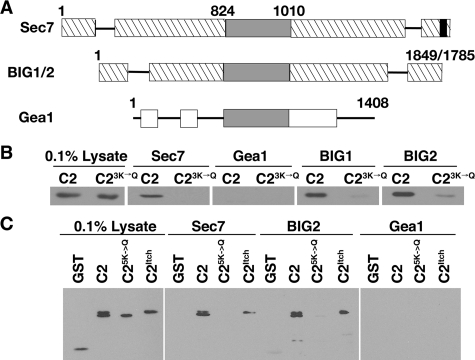
The Interaction between Sec7 and Rsp5 Is Conserved among Homologues—Both Sec7 and Rsp5 are conserved proteins with mammalian homologues. Sec7 homologues serve as Arf GEFs on various membranes in the cell and are characterized by a catalytic Sec7 domain. The Sec7 domain family includes two large GEF families represented by Sec7/BIG proteins and Gea/GNOM proteins (Fig. 3A, reviewed in Ref. 18). As this classification suggests, yeast Sec7 is more closely related to mammalian BIG proteins (28% identical, 49% similar) than to the yeast Gea proteins (18% identical, 36% similar). We inserted DNA encoding the C-terminal regions of BIG1, BIG2, and Gea1 that most closely aligned with Sec7 amino acids 1836–2009 into bacterial expression plasmids. These His6-tagged C-terminal fragments were expressed in bacteria, immobilized on beads, and incubated with yeast lysates prepared from cells expressing either GST-C2 or GST-C23K→Q. The C2 domain bound to the BIG1 and BIG2 C termini, but not to the Gea1 C terminus. Binding to BIG1 and BIG2 was markedly reduced by the C23K→Q mutation (Fig. 3B). These observations indicate that the Rsp5 C2 domain can bind to the C terminus of closely related Sec7 homologues.
FIGURE 3.
The interaction between Rsp5 and Sec7 is conserved among homologues. A, schematic of the ARF GEFs S. cerevisiae Sec7, human BIG1/2, and S. cerevisiae Gea1. The gray boxes represent the Sec7 catalytic domain and the black box represents the CIR site. Hatched boxes represent regions that are homologous within the Sec7/BIG family. White boxes represent regions that are homologous within the Gea/GNOM family (23). B, bacterial lysates containing GST-C2 and GST-C23K→Q were incubated with immobilized His6-tagged C termini of Sec7, BIG1, BIG2, and Gea1. Bound proteins were eluted by boiling, resolved by SDS-PAGE, and analyzed by immunoblotting with GST antibodies. The presence of equal amounts of immobilized protein in each reaction was verified by SDS-PAGE and Coomassie staining (not shown). C, lysates prepared from bacteria expressing GST, GST-C2, GST-C23K→Q, and GST-ItchC2 were incubated with immobilized His6-tagged C termini of Sec7 and BIG2. Bound proteins were eluted by boiling, resolved by SDS-PAGE, and analyzed by immunoblotting with GST antibodies. His6-Gea1 was used as a control for nonspecific binding. The presence of equal amounts of immobilized protein in each reaction was verified by SDS-PAGE and Coomassie staining (not shown).
A mammalian member of the Nedd4/Rsp5 ubiquitin ligase family is Itch, the mouse homologue of AIP4 (30, 60, 61). Itch/AIP4 is localized to endosomes and the TGN. On endosomal membranes, Itch/AIP4 functions in the ubiquitination of endosomal sorting machinery (62, 63). We expressed the C2 domain of Itch as a GST fusion protein in E. coli and incubated lysates prepared from these bacteria with the Sec7, BIG2, and Gea1 C termini immobilized on beads. Both the Rsp5 and Itch C2 domains bound to the BIG2 C terminus, but not to the Gea1 C terminus (Fig. 3C). Furthermore, the Itch C2 domain bound to the C terminus of Sec7. The observed interactions between Sec7, Rsp5, and their homologues suggest that the Sec7/BIG GEF proteins bind to Nedd4/Rsp5 family ubiquitin ligases in higher eukaryotes.
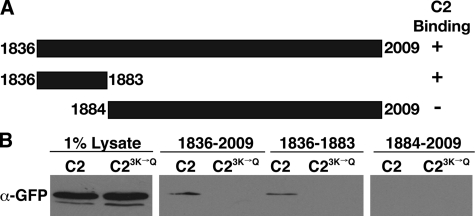
Identification and Characterization of the C2 Domain-binding Site in the Sec7 C Terminus—Because the Rsp5 C2 domain-Sec7 interaction is conserved and therefore likely to be functionally important, we further defined the C2 domain-binding site on Sec7. From plasmids isolated in the yeast two-hybrid screen, we deduced that the region encoding amino acids 1836–2009 of Sec7 was involved in binding to the C2 domain. We made truncations of DNA encoding this region in bacterial expression plasmids (Fig. 4A). These Sec7 fragments were expressed and immobilized on beads. Because there was high background binding of bacterially expressed GST fusion proteins to these immobilized Sec7 fragments, we incubated the Sec7 fragments with yeast lysates containing a GFP-tagged C2 domain (GFP-C2) or GFP-C23K→Q. The Sec7-(1836–1883) fragment bound to the C2 domain, whereas the Sec7-(1884–2009) fragment did not, and the 3K→Q mutation significantly reduced or eliminated this interaction (Fig. 4B). This observation indicates that amino acids 1836–1883 of Sec7 are sufficient to bind to the Rsp5 C2 domain. Hereafter, we refer to this Sec7 sequence as the C2 domain interacting region (CIR). BIG1 and BIG2 carry sequences in the corresponding regions of their C termini that are 20% identical and 45% similar to the CIR.
FIGURE 4.
Sec7 amino acids 1836–1883 are sufficient for interaction with the C2 domain of Rsp5. A, schematic of Sec7 C terminus fragments. The ability of each fragment to bind the C2 domain is indicated on the right. B, yeast lysates containing GFP-C2 (LHY4488) or GFP-C23K→Q (LHY4007) were incubated with immobilized His6-Sec7 fragments. Bound proteins were eluted by boiling, resolved by SDS-PAGE, and analyzed by immunoblotting with GFP antibodies. The presence of equal amounts of immobilized proteins in each reaction was verified by SDS-PAGE and Coomassie staining (not shown).
Because Sec7 is an essential protein, we investigated the role of the Sec7 CIR in cell viability. We integrated DNA encoding GFP-tagged Sec7 (Sec7-GFP) or Sec7ΔCIR (Sec7ΔCIR-GFP) into the genome in place of endogenous SEC7. The integration events were confirmed by PCR analysis of genomic DNA isolated from transformants and equal expression of the GFP-tagged proteins was verified by immunoblotting. sec7ΔCIR-GFP integrants were alive and showed no observable growth defect at 37 °C on rich medium or media supplemented with 6 or 8 mm caffeine (data not shown). We conclude that the CIR of Sec7 is not required for the essential role of Sec7 as an Arf GEF.
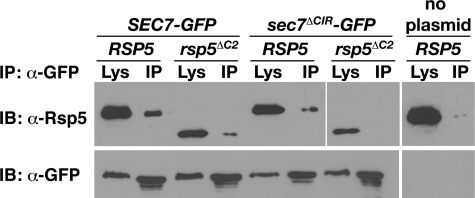
Characterization of the Cellular Interaction between Sec7 and Rsp5—To determine whether the interaction between Sec7 and Rsp5 in vivo is dependent on the Sec7 CIR and the Rsp5 C2 domain, we performed native co-immunoprecipitation experiments. For these experiments, we used an rsp5 mutant carrying a complete deletion of the C2 domain, rsp5ΔC2, rather than rsp53K→Q, so that our analysis would not be complicated by indirect, C2-domain-mediated Rsp5 interactions that might occur in the cell. Sec7-GFP and Sec7ΔCIR-GFP were precipitated with anti-GFP from lysates of cells expressing either Rsp5 or Rsp5ΔC2, and immunoblots of these precipitates were probed with Rsp5 antiserum. In the absence of the C2 domain, there was a decrease in the amount of Rsp5 precipitated with Sec7, although the interaction was not completely abolished (Fig. 5). Similar to deletion of the C2 domain from Rsp5, deletion of the Sec7 CIR caused a partial decrease in the interaction between Rsp5 and Sec7. Surprisingly, however, deletion of both the C2 domain and the CIR abolished the Sec7-Rsp5 interaction (Fig. 5). Similar amounts of Sec7-GFP and Sec7ΔCIR-GFP were immunoprecipitated from each lysate and both Rsp5 and Rsp5ΔC2 were expressed at a high level. Therefore, the observed decreases in Rsp5 and Rsp5ΔC2 co-precipitated were likely the result of a diminished interaction between Rsp5 and Sec7 and not to variation in mutant protein expression levels. These observations suggest that a C2 domain-CIR interaction is important for binding of Rsp5 to Sec7, but that other interactions also contribute to binding.
FIGURE 5.
The Sec7-Rsp5 interaction requires both the C2 domain and CIR. Lysates prepared from RSP5 or rsp5ΔC2 cells expressing Sec7-GFP (LHY5466, LHY5467), Sec7ΔCIR-GFP (LHY5518, LHY5519), or untagged Sec7 (LHY1107) were subjected to immunoprecipitation (IP) with GFP antibodies. Total lysates (anti-GFP blots, 0.7%; RSP5 anti-Rsp5 blots, 0.07%; rsp5ΔC2 anti-Rsp5 blots, 0.14%) and precipitated proteins were resolved by SDS-PAGE and analyzed by immunoblotting (IB) with GFP and Rsp5 antibodies.
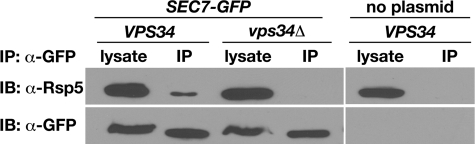
Role of Phosphoinositides in the Sec7-Rsp5 Interaction—Previously we showed that the Rsp5 C2 domain binds to PI(3)P (43). To investigate the role of PI(3)P in the Sec7-Rsp5 interaction, we analyzed cells that lack Vps34, the sole PI 3-kinase in yeast. vps34Δ cells are severely deficient in PI(3)P and are defective in vacuolar protein sorting (64–67). Sec7-GFP was immunoprecipitated using anti-GFP from lysates prepared from VPS34 and vps34Δ cells. Rsp5 co-precipitated with Sec7-GFP from VPS34 lysates, but not from vps34Δ lysates (Fig. 6), indicating that Vps34, and possibly PI(3)P, is required for an interaction between Sec7 and Rsp5 to occur.
FIGURE 6.
Vps34 is required for the interaction of Sec7 and Rsp5 in vivo. Lysates prepared from VPS34 (LHY5440) or vps34Δ (LHY5508) cells expressing Sec7-GFP, or VPS34 cells expressing untagged Sec7 (LHY1850), were subjected to immunoprecipitation (IP) with GFP antibodies. Total lysates (0.7%, anti-GFP blot and 0.07%, anti-Rsp5 blot) and precipitated proteins were resolved by SDS-PAGE and analyzed by immunoblotting (IB) with GFP and Rsp5 antibodies.
Physiological Functions of the Sec7 CIR Motif—Sec7 is required for the transport of proteins from the Golgi to the vacuole (19, 21, 22) and the Rsp5 C2 domain is required for the sorting of biosynthetic cargo into MVE vesicles en route to the vacuole (43–45). Therefore we tested whether the CIR of Sec7 was required to transport several different cargos along the TGN → vacuole pathway. Both GFP-CPS (ubiquitin-dependent cargo) and carboxypeptidase Y (ubiquitin-independent cargo) were transported normally to the vacuole lumen in sec7ΔCIR cells (data not shown). Regulated ubiquitin-dependent trafficking of plasma membrane proteins (e.g. Gap1-GFP) from the TGN to the vacuole was also normal in sec7ΔCIR cells (data not shown).
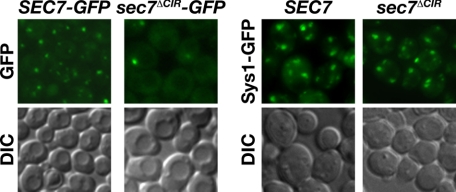
We next investigated the role of the CIR in Sec7 localization. Sec7-GFP and Sec7ΔCIR-GFP were visualized by fluorescence microscopy. Sec7 was observed primarily in three to four punctate spots per cell, structures that were shown previously to correspond to the Golgi (51, 68–73). By contrast, Sec7ΔCIR-GFP was observed in fewer punctate spots, and instead had increased cytoplasmic localization (Fig. 7). To determine whether the mislocalization of Sec7ΔCIR was due to a CIR-dependent Sec7-Golgi interaction or whether the ΔCIR deletion disrupted overall Golgi structure, we analyzed the localization of Sys1-GFP, a Golgi protein that colocalizes with Sec7 (72). In both SEC7 and sec7ΔΔCIR cells, Sys1-GFP was found in three to four punctate spots (Fig. 7), indicating that general Golgi morphology is normal in the absence of the CIR and that the CIR is likely to mediate a specific association of Sec7 with Golgi membranes.
FIGURE 7.
Localization of Sec7 to the Golgi is dependent the CIR. The localization of Sec7-GFP (LHY5466), Sec7ΔCIR-GFP (LHY5518), and Sys1-GFP in SEC7 (LHY5604) and sec7ΔCIR (LHY5605) cells was visualized by fluorescence microscopy and DIC microscopy.
DISCUSSION
In this study we identified a direct interaction between the Rsp5 C2 domain and a 50-amino acid region of the Sec7 C terminus, termed the CIR. This is likely to be a biologically important interaction because it is conserved between Rsp5 and Sec7 homologues. Intriguingly, this interaction requires three lysine residues in the C2 domain that are also involved in mediating interactions with phosphoinositides (43). Therefore, it is possible that phosphoinositides and Sec7 compete for binding to the same surface on the C2 domain. C2 domains are able to interact with both proteins and lipids (37, 38, 42, 54, 74, 75), however, competition between proteins and lipids for binding C2 domains has not been investigated. Rsp5 is able to form homodimers (48), suggesting the possibility that two C2 domains of an Rsp5 dimer bind different ligands, forming a network of interactions.
The CIR is a unique sequence that does not have motifs in common with any currently characterized protein domains. There is homology between Sec7 and BIG1/2 in this region (20% identity, 45% similarity), suggesting that this interaction may be specific to the Sec7/BIG subfamily of Sec7 domain-containing proteins. Although other regions of homology have been characterized in this family (reviewed in Ref. 18), none have been found that include the extreme C terminus or CIR. Further investigation into specific CIR residues required for binding to the Rsp5 C2 domain is necessary to define the minimal, conserved CIR motif.
We observed a significant, but partial, decrease in Sec7-Rsp5 binding in the absence of the CIR or C2 domain. Therefore, in addition to direct binding of C2 domain lysines to the CIR, other interactions mediate binding of Sec7 to Rsp5 in vivo. These interactions could be direct and involve other parts of the proteins. However, it is more likely that indirect interactions occur, mediated through other binding partners present in the yeast cell.
Vps34 is also required for the Sec7-Rsp5 interaction. Because Vps34 is a PI 3-kinase, the Sec7-Rsp5 interaction may require either the Vps34 protein or the production of PI(3)P by Vps34, to associate efficiently. The Vps34 protein is involved in the recruitment of proteins to endosomal membranes for retrograde endosome to TGN trafficking and Sec7 has been found to accumulate in endosomal compartments in vps34Δ cells (76–78). Thus, perhaps Sec7 and Rsp5 cannot associate in vps34Δ cells because the proteins are segregated to different subcellular locations or organelle subdomains due to defects in retrograde trafficking. Alternatively, PI(3)P might enhance Rsp5-Sec7 interaction by providing a microdomain for Rsp5 and Sec7 colocalization.
Sec7 overexpression suppresses the growth and GFP-CPS sorting phenotypes of an rsp5 mutant with a defective C2 domain (rsp55K→Q), indicating a functional relationship between the two proteins. The C25K→Q mutant cannot bind well to either the Sec7 CIR sequence or to PI(3)P (this manuscript and Ref. 43). Disrupting only the C2-CIR interaction is unlikely to be deleterious because the sec7ΔCIR mutation does not confer a detectable phenotype. However, an Rsp5-Sec7 interaction mediated by the CIR domain, or other parts of Sec7, may be crucial if Rsp5 is unable to bind PI(3)P. Thus, the higher cellular concentration of Sec7 provided by overexpression might overcome the defects caused by the rsp55K→Q mutation directly by enhancing Rsp5-Sec7 interaction, or indirectly by stimulating the Golgi function in which Rsp5 participates.
As mentioned above, deletion of the CIR sequence from Sec7 did not cause a growth phenotype. It also did not affect TGN → vacuole trafficking of multiple cargoes. Perhaps the CIR is required for trafficking of specific cargo to the vacuole via an as yet unidentified mechanism. It is also possible that the CIR functions redundantly with other proteins or domains within Sec7. Redundancy is likely, because the CIR deletion only partially inhibited Rsp5-Sec7 interaction. It is also possible that a CIR-C2 domain interaction is required for Sec7 ubiquitination, although we were unable to detect ubiquitinated Sec7 (data not shown). Interactions between Sec7/BIG GEFs and Nedd4/Rsp5 family ubiquitin ligases are conserved. The C terminus of BIG2 binds to the C2 domain of Itch. Itch and BIG2 both localize to endosomal membranes (24, 63), consistent with the idea that they might also interact in vivo. Even though deletion of the CIR sequence did not allow us to define a definitive role for the Rsp5-Sec7 interaction, the conservation of the interaction between Rsp5 and Sec7 homologues suggest it is likely to be functionally important.
The CIR is important for Sec7 localization, as indicated by the increased cytoplasmic localization of Sec7 lacking the CIR. Large Arf GEFs are recruited to the TGN independently of interactions with Arf proteins (79), although little else is known about how recruitment occurs. PI(3)P may recruit Sec7 to sites of retrograde trafficking via an adaptor protein that interacts with the CIR. CIR-binding proteins enriched on PI(3)P containing membranes would serve as a mechanism to retrieve Sec7 from endosomal compartments back to the TGN and promote further vesicle fission from the TGN. Interestingly, Sec7 binds to an uncharacterized membrane-anchored protein that might serve this function (80).
Although Sec7 is a large protein (2,009 amino acids), few binding partners have been identified. A central fragment of the protein containing the catalytic Sec7 domain binds to Arf. A large C-terminal fragment of Sec7 (aa 1197–2009) binds directly or indirectly to the Sec23 and Sec24 components of the COPII coat (21). Here we identify a conserved, novel interaction of Sec7/BIG Arf GEFs and Nedd4/Rsp5 ubiquitin ligases that requires the PI 3-kinase Vps34. Furthermore, we identify a region near the Sec7 C terminus that participates in binding to the C2 domain of the Rsp5 ubiquitin ligase and is required for Sec7 localization, referred to as CIR. Coordination of cargo or machinery ubiquitination and Arf activation may facilitate the efficient transport of proteins that leave the TGN destined for the vacuole.
Acknowledgments
We are grateful to Nava Segev, Scott Emr, Martha Vaughan, Kazuhisa Nakayama, Ben Glick, and Robert Piper for providing reagents, and Eric Weiss and Heike Fölsch for use of equipment. We gratefully acknowledge the use of microscopes in the Biological Imaging Facilities at Northwestern University. This article was improved by comments from Ingrid Jordens and Shelby King.
This work was supported, in whole or in part, by National Institutes of Health Grants DK53257 and DK61299 (to L. H.) and Training Grant T32GM08061 (to D. A. K. D.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TGN, trans-Golgi network; GFP, green fluorescent protein; CIR, C2 domain interacting region; GEF, guanine nucleotide exchange factor; PI(3)P, phosphatidylinositol 3-phosphate; GST, glutathione S-transferase; CPS, carboxypeptidase S; MVE, multivesicular endosome; PI 3-kinase, phosphoinositide 3-kinase; aa, amino acid; IP, immunoprecipitation; MES, 4-morpholineethanesulfonic acid.
D. A. Klos Dehring, W. Lin, and L. Hicke, unpublished data.
A. Alder and L. Hicke, unpublished data.
References
- 1.Gu, F., Crump, C. M., and Thomas, G. (2001) Cell Mol. Life Sci. 58 1067-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Boulan, E., and Musch, A. (2005) Biochim. Biophys. Acta 1744 455-464 [DOI] [PubMed] [Google Scholar]
- 3.Piper, R. C., and Luzio, J. P. (2007) Curr. Opin. Cell Biol. 19 459-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicke, L., and Dunn, R. (2003) Annu. Rev. Cell Dev. Biol. 19 141-172 [DOI] [PubMed] [Google Scholar]
- 5.Shearwin-Whyatt, L., Dalton, H. E., Foot, N., and Kumar, S. (2006) Bioessays 28 617-628 [DOI] [PubMed] [Google Scholar]
- 6.Pak, Y., Glowacka, W. K., Bruce, M. C., Pham, N., and Rotin, D. (2006) J. Cell Biol. 175 631-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNatt, M. W., McKittrick, I., West, M., and Odorizzi, G. (2007) Mol. Biol. Cell 18 697-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oestreich, A. J., Aboian, M., Lee, J., Azmi, I., Payne, J., Issaka, R., Davies, B. A., and Katzmann, D. J. (2007) Mol. Biol. Cell 18 707-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stawiecka-Mirota, M., Pokrzywa, W., Morvan, J., Zoladek, T., Haguenauer-Tsapis, R., Urban-Grimal, D., and Morsomme, P. (2007) Traffic 8 1280-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson, H., and Bonifacino, J. S. (2007) Mol. Biol. Cell 18 1781-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piper, R. C., and Katzmann, D. J. (2007) Annu. Rev. Cell Dev. Biol. 23 519-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley, J. H. (2008) Curr. Opin. Cell Biol. 20 4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss, J., and Vaughan, M. (1998) J. Biol. Chem. 273 21431-21434 [DOI] [PubMed] [Google Scholar]
- 14.Donaldson, J. G. (2005) Biochem. Soc. Trans. 33 1276-1278 [DOI] [PubMed] [Google Scholar]
- 15.Zhu, Y., Traub, L. M., and Kornfeld, S. (1998) Mol. Biol. Cell 9 1323-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takatsu, H., Yoshino, K., Toda, K., and Nakayama, K. (2002) Biochem. J. 365 369-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson, C. L., and Casanova, J. E. (2000) Trends Cell Biol. 10 60-67 [DOI] [PubMed] [Google Scholar]
- 18.Casanova, J. E. (2007) Traffic 8 1476-1485 [DOI] [PubMed] [Google Scholar]
- 19.Achstetter, T., Franzusoff, A., Field, C., and Schekman, R. (1988) J. Biol. Chem. 263 11711-11717 [PubMed] [Google Scholar]
- 20.Franzusoff, A., Lauze, E., and Howell, K. E. (1992) Nature 355 173-175 [DOI] [PubMed] [Google Scholar]
- 21.Deitz, S. B., Rambourg, A., Kepes, F., and Franzusoff, A. (2000) Traffic 1 172-183 [DOI] [PubMed] [Google Scholar]
- 22.Katzmann, D. J., Babst, M., and Emr, S. D. (2001) Cell 106 145-155 [DOI] [PubMed] [Google Scholar]
- 23.Shin, H. W., and Nakayama, K. (2004) J. Biochem. (Tokyo) 136 761-767 [DOI] [PubMed] [Google Scholar]
- 24.Shin, H. W., Morinaga, N., Noda, M., and Nakayama, K. (2004) Mol. Biol. Cell 15 5283-5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, H., Adamik, R., Pacheco-Rodriguez, G., Moss, J., and Vaughan, M. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 1627-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, K. F., Shen, X., Li, H., Pacheco-Rodriguez, G., Moss, J., and Vaughan, M. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2784-2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizaki, R., Shin, H. W., Iguchi-Ariga, S. M., Ariga, H., and Nakayama, K. (2006) Genes Cells 11 949-959 [DOI] [PubMed] [Google Scholar]
- 28.Ramaen, O., Joubert, A., Simister, P., Belgareh-Touze, N., Olivares-Sanchez, M. C., Zeeh, J. C., Chantalat, S., Golinelli-Cohen, M. P., Jackson, C. L., Biou, V., and Cherfils, J. (2007) J. Biol. Chem. 282 28834-28842 [DOI] [PubMed] [Google Scholar]
- 29.Ishizaki, R., Shin, H. W., Mitsuhashi, H., and Nakayama, K. (2008) Mol. Biol. Cell 19 2650-2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotin, D., Staub, O., and Haguenauer-Tsapis, R. (2000) J. Membr. Biol. 176 1-17 [DOI] [PubMed] [Google Scholar]
- 31.d'Azzo, A., Bongiovanni, A., and Nastasi, T. (2005) Traffic 6 429-441 [DOI] [PubMed] [Google Scholar]
- 32.Ingham, R. J., Gish, G., and Pawson, T. (2004) Oncogene 23 1972-1984 [DOI] [PubMed] [Google Scholar]
- 33.Nalefski, E. A., and Falke, J. J. (1996) Protein Sci. 5 2375-2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizo, J., and Südhof, T. C. (1998) J. Biol. Chem. 273 15879-15882 [DOI] [PubMed] [Google Scholar]
- 35.Cho, W., and Stahelin, R. V. (2006) Biochim. Biophys. Acta 1761 838-849 [DOI] [PubMed] [Google Scholar]
- 36.Cho, W. (2001) J. Biol. Chem. 276 32407-32410 [DOI] [PubMed] [Google Scholar]
- 37.Plant, P. J., Yeger, H., Staub, O., Howard, P., and Rotin, D. (1997) J. Biol. Chem. 272 32329-32336 [DOI] [PubMed] [Google Scholar]
- 38.Plant, P. J., Lafont, F., Lecat, S., Verkade, P., Simons, K., and Rotin, D. (2000) J. Cell Biol. 149 1473-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecchione, A., Marchese, A., Henry, P., Rotin, D., and Morrione, A. (2003) Mol. Cell Biol. 23 3363-3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavsak, P., Rasmussen, R. K., Causing, C. G., Bonni, S., Zhu, H., Thomsen, G. H., and Wrana, J. L. (2000) Mol. Cell 6 1365-1375 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki, C., Murakami, G., Fukuchi, M., Shimanuki, T., Shikauchi, Y., Imamura, T., and Miyazono, K. (2002) J. Biol. Chem. 277 39919-39925 [DOI] [PubMed] [Google Scholar]
- 42.Wiesner, S., Ogunjimi, A. A., Wang, H. R., Rotin, D., Sicheri, F., Wrana, J. L., and Forman-Kay, J. D. (2007) Cell 130 651-662 [DOI] [PubMed] [Google Scholar]
- 43.Dunn, R., Klos, D. A., Adler, A. S., and Hicke, L. (2004) J. Cell Biol. 165 135-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katzmann, D. J., Sarkar, S., Chu, T., Audhya, A., and Emr, S. D. (2004) Mol. Biol. Cell 15 468-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morvan, J., Froissard, M., Haguenauer-Tsapis, R., and Urban-Grimal, D. (2004) Traffic 5 383-392 [DOI] [PubMed] [Google Scholar]
- 46.Roth, M. G. (2004) Physiol. Rev. 84 699-730 [DOI] [PubMed] [Google Scholar]
- 47.Sherman, F. (1991) Methods Enzymol. 194 3-21 [DOI] [PubMed] [Google Scholar]
- 48.Dunn, R., and Hicke, L. (2001) Mol. Biol. Cell 12 421-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones, S., Jedd, G., Kahn, R. A., Franzusoff, A., Bartolini, F., and Segev, N. (1999) Genetics 152 1543-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamenova, S. D., Dunn, R., Adler, A. S., and Hicke, L. (2004) J. Biol. Chem. 279 16017-16025 [DOI] [PubMed] [Google Scholar]
- 51.Seron, K., Tieaho, V., Prescianotto-Baschong, C., Aust, T., Blondel, M.-O., Guillaud, P., Devilliers, G., Rossanese, O. W., Glick, B. S., Riezman, H., Keranen, S., and Haguenauer-Tsapis, R. (1998) Mol. Biol. Cell 9 2837-2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James, P., Halladay, J., and Craig, E. A. (1996) Genetics 144 1425-1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shih, S. C., Katzmann, K. J., Schnell, J. D., Sutanto, M., Emr, S. C., and Hicke, L. H. (2002) Nat. Cell Biol. 4 389-393 [DOI] [PubMed] [Google Scholar]
- 54.Morrione, A., Plant, P., Valentinis, B., Staub, O., Kumar, S., Rotin, D., and Baserga, R. (1999) J. Biol. Chem. 274 24094-24099 [DOI] [PubMed] [Google Scholar]
- 55.Ochoa, W. F., Torrecillas, A., Fita, I., Verdaguer, N., Corbalan-Garcia, S., and Gomez-Fernandez, J. C. (2003) Biochemistry 42 8774-8779 [DOI] [PubMed] [Google Scholar]
- 56.Grass, I., Thiel, S., Honing, S., and Haucke, V. (2004) J. Biol. Chem. 279 54872-54880 [DOI] [PubMed] [Google Scholar]
- 57.Benes, C. H., Wu, N., Elia, A. E., Dharia, T., Cantley, L. C., and Soltoff, S. P. (2005) Cell 121 271-280 [DOI] [PubMed] [Google Scholar]
- 58.Kutateladze, T. G., Ogburn, K. D., Watson, W. T., de Beer, T., Emr, S. D., Burd, C. G., and Overduin, M. (1999) Mol. Cell 3 805-811 [DOI] [PubMed] [Google Scholar]
- 59.Odorizzi, G., Babst, M., and Emr, S. D. (1998) Cell 95 847-858 [DOI] [PubMed] [Google Scholar]
- 60.Snyder, P. M., Price, M. P., McDonald, F. J., Adams, C. M., Volk, K. A., Zeiher, B. G., Stokes, J. B., and Welsh, M. J. (1995) Cell 83 969-978 [DOI] [PubMed] [Google Scholar]
- 61.Flores, S. Y., Debonneville, C., and Staub, O. (2003) Pflugers Arch. 446 334-338 [DOI] [PubMed] [Google Scholar]
- 62.Marchese, A., Raiborg, C., Santini, F., Keen, J. H., Stenmark, H., and Benovic, J. L. (2003) Dev. Cell 5 709-722 [DOI] [PubMed] [Google Scholar]
- 63.Angers, A., Ramjaun, A. R., and McPherson, P. S. (2004) J. Biol. Chem. 279 11471-11479 [DOI] [PubMed] [Google Scholar]
- 64.Herman, P. K., and Emr, S. D. (1990) Mol. Cell Biol. 10 6742-6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schu, P. V., Takegawa, K., Fry, M. J., Stack, J. H., Waterfield, M. D., and Emr, S. D. (1993) Science 260 88-91 [DOI] [PubMed] [Google Scholar]
- 66.Stack, J. H., and Emr, S. D. (1993) Curr. Opin. Cell Biol. 5 641-646 [DOI] [PubMed] [Google Scholar]
- 67.Stack, J. H., and Emr, S. D. (1994) J. Biol. Chem. 269 31552-31562 [PubMed] [Google Scholar]
- 68.Franzusoff, A., Redding, K., Crosby, J., Fuller, R. S., and Schekman, R. (1991) J. Cell Biol. 112 27-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossanese, O. W., Soderholm, J., Bevis, B. J., Sears, I. B., O'Connor, J., Williamson, E. K., and Glick, B. S. (1999) J. Cell Biol. 145 69-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao, X., Lasell, T. K., and Melancon, P. (2002) Mol. Biol. Cell 13 119-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., and Weissman, J. S. (2003) Nature 425 737-741 [DOI] [PubMed] [Google Scholar]
- 72.Losev, E., Reinke, C. A., Jellen, J., Strongin, D. E., Bevis, B. J., and Glick, B. S. (2006) Nature 441 1002-1006 [DOI] [PubMed] [Google Scholar]
- 73.Matsuura-Tokita, K., Takeuchi, M., Ichihara, A., Mikuriya, K., and Nakano, A. (2006) Nature 441 1007-1010 [DOI] [PubMed] [Google Scholar]
- 74.Zhang, J. Z., Davletov, B. A., Südhof, T. C., and Anderson, R. G. W. (1994) Cell 78 751-760 [DOI] [PubMed] [Google Scholar]
- 75.Sugita, S., Hata, Y., and Sudhof, T. C. (1996) J. Biol. Chem. 271 1262-1265 [DOI] [PubMed] [Google Scholar]
- 76.Kihara, A., Noda, T., Ishihara, N., and Ohsumi, Y. (2001) J. Cell Biol. 152 519-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burda, P., Padilla, S. M., Sarkar, S., and Emr, S. D. (2002) J. Cell Sci. 115 3889-3900 [DOI] [PubMed] [Google Scholar]
- 78.Demmel, L., Gravert, M., Ercan, E., Habermann, B., Muller-Reichert, T., Kukhtina, V., Haucke, V., Baust, T., Sohrmann, M., Kalaidzidis, Y., Klose, C., Beck, M., Peter, M., and Walch-Solimena, C. (2008) Mol. Biol. Cell 19 1991-2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beraud-Dufour, S., Paris, S., Chabre, M., and Antonny, B. (1999) J. Biol. Chem. 274 37629-37636 [DOI] [PubMed] [Google Scholar]
- 80.Wolf, J. R., Lasher, R. S., and Franzusoff, A. (1996) Biochem. Biophys. Res. Commun. 224 126-133 [DOI] [PubMed] [Google Scholar]