Abstract
Karyotype evolution in species with identical chromosome number but belonging to distinct phylogenetic clades is a long-standing question of plant biology, intractable by conventional cytogenetic techniques. Here, we apply comparative chromosome painting (CCP) to reconstruct karyotype evolution in eight species with x=7 (2n=14, 28) chromosomes from six Brassicaceae tribes. CCP data allowed us to reconstruct an ancestral Proto-Calepineae Karyotype (PCK; n=7) shared by all x=7 species analyzed. The PCK has been preserved in the tribes Calepineae, Conringieae, and Noccaeeae, whereas karyotypes of Eutremeae, Isatideae, and Sisymbrieae are characterized by an additional translocation. The inferred chromosomal phylogeny provided compelling evidence for a monophyletic origin of the x=7 tribes. Moreover, chromosomal data along with previously published gene phylogenies strongly suggest the PCK to represent an ancestral karyotype of the tribe Brassiceae prior to its tribe-specific whole-genome triplication. As the PCK shares five chromosomes and conserved associations of genomic blocks with the putative Ancestral Crucifer Karyotype (n=8) of crucifer Lineage I, we propose that both karyotypes descended from a common ancestor. A tentative origin of the PCK via chromosome number reduction from n=8 to n=7 is outlined. Comparative chromosome maps of two important model species, Noccaea caerulescens and Thellungiella halophila, and complete karyotypes of two purported autotetraploid Calepineae species (2n=4x=28) were reconstructed by CCP.
INTRODUCTION
Comparative linkage mapping of shared genetic markers reveals the extent of interspecies chromosome collinearity, as shown for grasses (Devos, 2005; Wei et al., 2007) and crucifers (reviewed by Koch and Kiefer, 2005). Cytogenetic evidence for collinear chromosome segments among closely related plants is difficult to obtain because ubiquitous and abundant dispersed repeats usually prevent sufficient chromosome specificity of DNA probes (Schubert et al., 2001). Crucifers (Brassicaceae) represent the only plant family to date in which homeologous chromosome regions/chromosomes have successfully been analyzed with chromosome-specific painting probes. Comparative chromosome painting (CCP) in Brassicaceae is feasible due to the availability of virtually repeat-free Arabidopsis thaliana BAC clones and the preferential location of highly repetitive DNA sequences at pericentromeric heterochromatin in most crucifer species. Therefore, CCP with selected repeat-free Arabidopsis BAC contigs can reveal collinear chromosome regions in other crucifer species (Jiang and Gill, 2006; Lysak and Lexer, 2006).
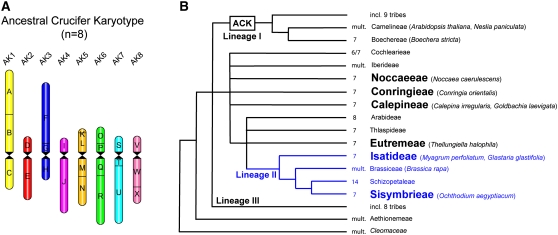
Although CCP reveals the extent of chromosome collinearity between A. thaliana and other species, it provides only limited information on the direction of karyotype evolution. To ascertain the evolutionary progression of karyotypic alterations, it is necessary to distinguish ancestral from derived karyotypes. Comparative genetic and cytogenetic analyses showed that the compact A. thaliana genome is characterized by a highly reshuffled and derived karyotype (Boivin et al., 2004; Kuittinen et al., 2004; Koch and Kiefer, 2005; Lysak et al., 2006), making this species inappropriate as a reference point for comparative studies across Brassicaceae (Schranz et al., 2006). Therefore, an Ancestral Crucifer Karyotype (ACK) with eight chromosomes (AK1 to AK8) and some 24 conserved genomic blocks (GBs; A to X) has been proposed (Figure 1A) (Lysak et al., 2006; Schranz et al., 2006). The ACK is based on the fact that x=8 is the most common base number in the family (Warwick and Al-Shehbaz, 2006) and that the eight chromosomes of Arabidopsis lyrata and Capsella rubella represent nearly identical linkage groups (Boivin et al., 2004; Kuittinen et al., 2004; Koch and Kiefer, 2005). Also n=6 and n=7 karyotypes share ancestral chromosomes with the ACK (Lysak et al., 2006). Moreover, the five studied extant karyotypes (n=5 to n=8) (Lysak et al., 2006) as well as the allopolyploid genome of Brassica napus (Parkin et al., 2005) and the ACK are composed of 24 evolutionarily conserved GBs (Schranz et al., 2006).
Figure 1.
ACK (n=8) and Phylogenetic Relationship of Selected Tribes and Species within the Family Brassicaceae.
(A) Scheme of the ACK of crucifer Lineage I comprising eight chromosomes (AK1 to AK8) and 24 GBs (A to X). Modified from Schranz et al. (2006).
(B) Three major phylogenetic lineages (Lineages I to III) were recognized within Brassicaceae (Beilstein et al., 2006). The six analyzed x=7 tribes (in boldface) are embedded within an unresolved assemblage of tribes including Lineage II (in blue). A tentative phylogenetic position of the ACK, and species analyzed and/or discussed in this study, are given. Base chromosome number (x) is indicated for each tribe (mult., multiple base numbers). The tree is modified from Koch and Al-Shehbaz (2008).
The current intrafamiliar classification of the family Brassicaceae (Al-Shehbaz et al., 2006), based on the ndhF phylogeny (Beilstein et al., 2006), includes at least 25 tribes clustered into three major phylogenetic lineages (Lineages I to III, with A. thaliana in Lineage I and Brassica in Lineage II) or having an unresolved position (Figure 1B). Genetic and cytogenetic analyses of cross-species chromosome homeology have mostly focused on close relatives of A. thaliana within Lineage I (Lysak et al., 2003, 2006; Koch and Kiefer, 2005; Schranz et al., 2007) and to a lesser extent on species of the tribe Brassiceae, belonging to Lineage II (Lysak et al., 2005; Parkin et al., 2005). Here we investigate (1) to what extent tribes of Lineage II and potentially affiliated tribes (Figure 1B), mostly possessing x=7 karyotypes, are congruous with the tentative ACK (n=8) of Lineage I; (2) whether the x=7 karyotypes descended from a common n=7 ancestral karyotype or originated repeatedly within this group of tribes (involving different rearrangements each time); and (3) to what degree collinear GBs are conserved among the chromosomes of x=7 species. Furthermore, we searched for clade- and tribe-specific chromosome rearrangements (cytogenetic signatures) that could help resolve the ambiguous tribal polytomy associated with Lineage II and affiliated x=7 tribes (Figure 1B) (Bailey et al., 2006; Beilstein et al., 2006; Koch et al., 2007; Koch and Al-Shehbaz, 2008).
To address the above questions, the pattern of karyotype evolution has been reconstructed for eight x=7 (2n=14, 28) species from six tribes by CCP with Arabidopsis BAC contigs arranged according to the eight ancestral chromosomes (AK1 to AK8) and 24 ancestral GBs (A to X) of the ACK. We analyzed three species of Lineage II (Glastaria glastifolia, Myagrum perfoliatum [both of tribe Isatideae], and Ochthodium aegyptiacum [assigned to Sisymbrieae; K. Mummenhoff, personal communication]) and five species of associated tribes (Calepina irregularis, Goldbachia laevigata [Calepineae], Conringia orientalis [Conringieae], Noccaea [Thlaspi] caerulescens [Noccaeeae], and Thellungiella halophila [=Eutrema salsugineum; Eutremeae]). Among these species, N. caerulescens is an important model of heavy metal accumulation (Peer et al., 2006) and T. halophila is an important model of salt and cold tolerance (Gong et al., 2005).
RESULTS
CCP Analysis Suggests a Common Ancestry of the x=7 Species and the ACK
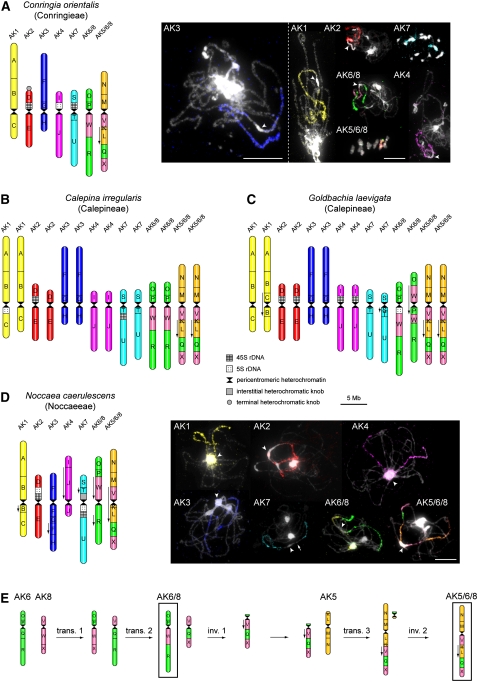
The revealed CCP patterns are here described in relation to the ACK (n=8; Figure 1A) as a reference genome. All 24 (A to X) conserved GBs of the ACK were unambiguously identified in chromosome complements of the analyzed species by CCP with A. thaliana BAC contigs as probes (see Methods and Supplemental Table 1 online). In C. orientalis (2n=2x=14; Conringieae), painting probes for ancestral chromosomes AK1 to AK4 and AK7 labeled a single pachytene bivalent or mitotic chromosome pair each (Figure 2A). C. irregularis and G. laevigata (2n=4x=28; Calepineae) showed the karyotype structure similar to that described for Conringia, but all five AK-like chromosomes were present in two copies due to a putative genome duplication (Figures 2B and 2C; see Supplemental Figure 1 online). Figure 2D shows the reconstructed karyotype of N. caerulescens (2n=2x=14; Noccaeeae). Similar to the pattern observed in Calepineae and Conringieae, five Noccaea homeologues resemble AK1 to AK4 and AK7 chromosomes. However, all but one AK-like chromosomes exhibit specific inversions (Figure 2D; see Supplemental Figure 2 online). The repatterning of AK1- and AK4-like homeologues was probably caused by two subsequent pericentric inversions, and the structure of the AK3-like chromosome can be explained by a paracentric and subsequent pericentric inversion (see Supplemental Figure 2 online). The AK7-like homeologue probably underwent a single pericentric inversion. The analyzed species from the three remaining tribes (all 2n=2x=14), M. perfoliatum and G. glastifolia (both Isatideae), O. aegyptiacum (Sisymbrieae), and T. halophila (Eutremeae), share four homeologues resembling the ancestral chromosomes AK1, AK3, AK4, and AK7 (Figures 3A and 3C to 3E).
Figure 2.
Reconstructed Karyotypes of Conringieae, Calepineae, and Noccaeeae Species.
(A) Karyotype of C. orientalis (2n=14; Conringieae) and chromosomes of this species revealed by CCP.
(B) Karyotype of C. irregularis (2n=28; Calepineae).
(C) Karyotype of G. laevigata (2n=28; Calepineae).
(D) Karyotype of N. caerulescens (2n=14; Noccaeeae) and Noccaea chromosomes after CCP.
(E) Tentative scenario of the origin of translocation chromosomes AK6/8 and AK5/6/8 from ancestral chromosomes AK5, AK6, and AK8.
The 24 GBs are indicated by uppercase letters (A to X) and colored according to their positions on chromosomes AK1 to AK8 of the ACK (Figure 1A). Downward-pointing arrows indicate the opposite orientation of GBs compared with the position in the ACK. Karyotypes in (A) to (D) are drawn to scale (bar = 5 Mb). In CCP images, arrowheads point to pericentromeric heterochromatin and the arrow indicates the heterochromatic knob. Bars = 10 μm.
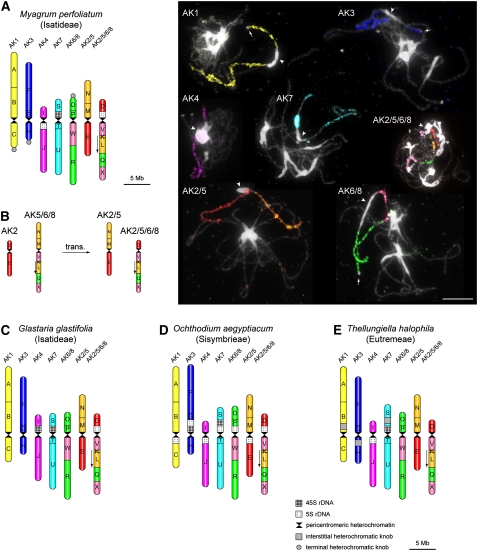
Figure 3.
Reconstructed Karyotypes of Eutremeae, Isatideae, and Sisymbrieae Species.
(A) Karyotype of M. perfoliatum (2n=14; Isatideae) and Myagrum chromosomes after CCP.
(B) A tentative scenario of the origin of translocation chromosomes AK2/5 and AK2/5/6/8 from chromosomes AK2 and AK5/6/8.
(C) Karyotype of G. glastifolia (2n=14; Isatideae).
(D) Karyotype of O. aegyptiacum (2n=14; Sisymbrieae).
(E) Karyotype of T. halophila (2n=14; Eutremeae).
The 24 GBs are indicated by uppercase letters (A to X) and colored according to their positions on chromosomes AK1 to AK8 of the ACK (Figure 1A). Downward-pointing arrows indicate the opposite orientation of GBs compared with the position in the ACK. In O. aegyptiacum, it was not possible to distinguish whether the 5S rDNA loci are located within a pericentromeric region of short or long arms of the analyzed mitotic chromosomes; thus, the 5S rDNA positions are only approximate. Karyotypes in (A) and (C) to (E) are drawn to scale (bar = 5 Mb). In CCP images, arrowheads refer to pericentromeric heterochromatin and arrows indicate heterochromatic knobs. Bar = 10 μm.
The identification of at least four AK-like homeologues and GB associations corresponding to those of the ACK across all analyzed tribes strongly suggests that the x=7 taxa and the ACK descended from a common ancestor. This was further corroborated by comparing the relative lengths of the 24 GBs among all eight species (see Supplemental Table 2 online) and with megabase size estimations for these segments in A. thaliana (see Supplemental Table 1 online). There was no significant difference between relative lengths of GBs among the analyzed taxa (analysis of variance; P = 0.008).
Structure and Origin of the Rearranged Chromosomes: the Proto-Calepineae Karyotype
Besides AK-like chromosomes, two chromosomes comprising novel associations of GBs were identified in the analyzed x=7 species. The most parsimonious origin of the two chromosomes (AK6/8 and AK5/6/8) has been reconstructed assuming that the two rearranged chromosomes arose as a result of chromosome number reduction from n=8 to n=7 and that the ancestral eight chromosomes resembled AK chromosomes of the ACK.
In Conringieae (C. orientalis) and Calepineae (C. irregularis and G. laevigata), chromosome AK6/8 consists of AK6-derived blocks O, P, and R, with the block W assigned to AK8. The AK6/8 chromosome probably originated from two subsequent reciprocal translocations between AK6 and AK8; the AK6/8 centromere was derived from AK6 or AK8 (Figure 2E). The initial translocation event led to a whole-arm exchange followed by a reciprocal translocation involving blocks R and X. The translocation chromosome V/Q/X became acrocentric via a pericentric inversion involving its short arm (block V). Then a reciprocal translocation transferred the long arm to the short arm end of AK5. The small translocation product (a minichromosome bearing the V/Q/X centromere) was apparently meiotically unstable and thus became eliminated. The AK5/6/8 was further modified by a paracentric inversion (Figure 2E). AK5/6/8 comprises the short arm and the centromere from AK5 (blocks M/N), whereas its long arm is composed of GBs from AK5 (K/L), AK6 (Q), and AK8 (V/X) (Figure 2). In Noccaeeae (N. caerulescens), AK6/8 and AK5/6/8 chromosomes were altered by secondary intrachromosomal rearrangements (Figure 2D). The structure of AK6/8 can be explained by a pericentric and a subsequent paracentric inversion, whereas the AK5/6/8 chromosome probably experienced a single pericentric inversion (see Supplemental Figure 2 online).
In Eutremeae, Isatideae, and Sisymbrieae (Figure 3), the AK6/8 chromosome is identical to that described in Conringieae, Calepineae, and Noccaeeae. The translocation chromosome AK2/5/6/8 bears blocks V/K/L/Q/X on its long arm, as in all other taxa; however, the short arm comprises block D of AK2. Blocks M/N (from AK5) and E (from AK2) constitute the complementary product (AK2/5) of a reciprocal translocation between AK5/6/8 and the ancestral chromosome AK2. The centromere identity in the two translocation chromosomes cannot be inferred from the present data (Figure 3B). In G. glastifolia, the AK2/5/6/8 chromosome is modified by a paracentric inversion on its long arm (Figure 3C).
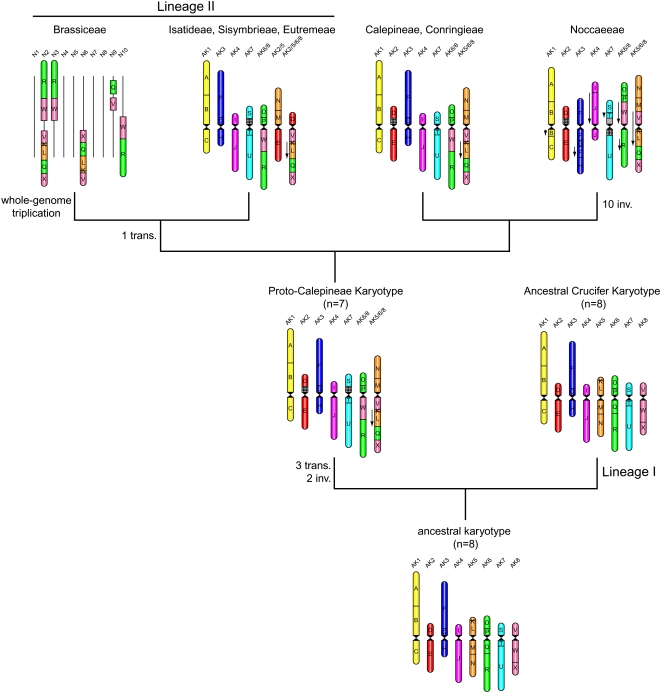
Based on the above data, a tentative Proto-Calepineae Karyotype (PCK; n=7) comprising five ancestral chromosomes (AK1 to AK4 and AK7) and two translocation chromosomes (AK6/8 and AK5/6/8) has been deduced. Following the most parsimonious interpretation, the PCK is likely the ancestor of all x=7 karyotypes analyzed (Figure 4).
Figure 4.
Reconstruction of Karyotype Evolution in Six x=7 Tribes and Brassiceae from the PCK (n=7) and an Ancestral Karyotype (n=8) Based on CCP Data.
The 24 GBs are indicated by uppercase letters (A to X) and colored according to their position on chromosomes AK1 to AK8 of the ACK (Figure 1A). Downward-pointing arrows indicate the opposite orientation of GBs compared with the position in the ACK. A tentative number of translocations (transl.) and inversions (inv.) is given at the nodes of the chromosomal phylogeny. 45S rDNA loci are shown as cross-hatched boxes. For the tribe Brassiceae (exemplified by the B. rapa karyotype, n=10; Parkin et al., 2005), only associations of GBs shared with the PCK are displayed; other chromosomes/chromosome regions are shown as black bars.
Comparative Analysis of Heterochromatic Chromosomal Landmarks
Chromosome homeology and chromosome rearrangements revealed by CCP have been further compared with prominent heterochromatin and repeat block landmarks of the analyzed species. Heterochromatic arrays of pachytene bivalents were identified by 4′,6-diamidino-2-phenylindole staining and by fluorescence in situ hybridization localization of 5S and 45S rDNA. Species-specific heterochromatin profiles were compared with estimated genome size values.
The number and position of rDNA loci found in the x=7 species are given in Table 1 and in Figures 2A to 2D, 3A, and 3C to 3E. In all species, 45S rDNA loci (NORs) have been detected adjacent to pericentromeric heterochromatic regions. In C. orientalis, C. irregularis, G. laevigata, and N. caerulescens, a 45S rDNA locus was consistently found adjacent to block D on the AK2-like chromosome and frequently on the AK7-like chromosome. In G. glastifolia, M. perfoliatum, O. aegyptiacum, and T. halophila, AK2 arms are separated by translocation and the 45S rDNA locus was no longer found to be associated with block D on chromosome AK2/5/6/8. Another locus is always associated with block S on the AK7-like homeologue. Hence, the location of NORs on chromosomes AK2 and AK7 may represent an ancestral condition of the PCK (Figure 4).
Table 1.
Chromosome Number, Monoploid Genome Size (Cx), and Number of rDNA Loci of the x=7 Species Analyzed
| Species | 2n | Cx (pg/Mb)a | No. of 45S rDNA Loci | No. of 5S rDNA Loci |
|---|---|---|---|---|
| Species with two translocation chromosomes | ||||
| Conringia orientalis | 14 | 0.228/223 | 4 | 2 |
| Calepina irregularis | 28 | 0.202/396 | 4 | 2 |
| Goldbachia laevigata | 28 | 0.218/427 | 8 | 2 |
| Noccaea caerulescens (=Thlaspi caerulescens) | 14 | 0.612/600 | 4 | 4 |
| Species with three translocation chromosomes | ||||
| Myagrum perfoliatum | 14 | 0.312/305 | 2 | 4 |
| Glastaria glastifolia | 14 | 0.425/416 | 4 | 2 |
| Ochthodium aegyptiacum | 14 | 0.336/329 | 4 | 14 |
| Thellungiella halophila (=Eutrema salsugineum) | 14 | 0.661/648 | 2 | 2 |
Cx values based on the Cx value of A. thaliana (0.16 pg/157 Mb).
The 5S rDNA loci are localized within pericentromeric heterochromatic regions of different homeologues in species of Conringieae, Calepineae, and Noccaeeae (Figures 2A to 2D). In tribes Eutremeae, Isatideae, and Sisymbrieae, the 5S rDNA is most frequently found on the short arm of chromosome AK2/5/6/8 (adjacent to block D) and/or on the long arm of AK4-like homeologue (Figures 3A and 3C to 3E). Remarkably, 5S rDNA loci are present within pericentromeric regions of all seven chromosome pairs in O. aegyptiacum (Figure 3D; see Supplemental Figure 3A online).
In tetraploid Calepineae species, rDNA loci were often located on only one of the two homeologues. In C. irregularis, both 5S and 45S rDNA loci were located on only one of the two homeologues (Figure 2B; see Supplemental Figure 1 online), whereas in G. laevigata, a 5S rDNA locus was situated within one of the two homeologues, while 45S rDNA loci occupied pericentromeric positions on both homeologues (Figure 2C; see Supplemental Figure 1 online).
Except for rDNA arrays, terminal and interstitial heterochromatic knobs were discerned in three species. In C. orientalis, a terminal knob is located on the short arm of AK2 homeologue (Figure 2A). Three terminal knobs (on AK1-like, AK3-like, and AK6/8 chromosomes) were revealed in M. perfoliatum (Figure 3A). In T. halophila, three large interstitial knobs are located close to centromeres of AK1-, AK3-, and AK7-like chromosomes (Figure 3E).
The monoploid genome size (Cx) varied from 0.20 pg (223 Mb) in C. orientalis to 0.66 pg (648 Mb) in T. halophila (Table 1). No apparent relationship was found between genome size variation and heterochromatin amount, chromosome homeology pattern, or the phylogenetic position of the x=7 species. Although the highest C value in T. halophila can be tentatively linked to the presence of three interstitial knobs and the large arrays of pericentromeric heterochromatin revealed in this species (see Supplemental Figure 3B online), a comparable genome size (0.61 pg/600 Mb) was estimated in N. caerulescens possessing only one knob and less extensive pericentromeric heterochromatic regions (Figure 2D).
DISCUSSION
We have reconstructed the karyotype evolution of eight x=7 species from six Brassicaceae tribes, thereby reconstructing by chromosome analyses the extent of whole-genome collinearity among a group of plant species for which no comparative cytogenetic and genetic maps were previously available. CCP using Arabidopsis BAC contigs arranged according to their position within the putative ACK of Lineage I (Lysak et al., 2006; Schranz et al., 2006) was applied to uncover homeologous chromosome regions of the x=7 species.
The ACK and the PCK
Five chromosomes shared between the ACK (n=8) and the PCK (n=7) unequivocally argue for a common origin of the two karyotypes (Figure 4). As the phylogenetic relationship between Lineage I and tribes affiliated with Lineage II remains largely unresolved (Koch and Al-Shehbaz, 2008) (Figure 1B), two alternative scenarios reconstructing the origin of the translocation chromosomes as well as the entire PCK complement have to be considered. The first assumes that a common ancestral karyotype for both clades (i.e., Lineages I and II) possessed eight chromosome pairs, and the second scenario implies a karyotype of seven chromosome pairs (n=7). Based on our current understanding of genome evolution in Brassicaceae, the former theoretical model advocating an ancestral n=8 karyotype for both lineages is preferred. The most parsimonious scenario of chromosome number reduction from n=8 to n=7 requires only three translocations and two inversions (Figure 2E), compared with a higher number of less parsimonious steps necessary to reconstruct the ACK from the PCK. Moreover, the latter model (i.e., n=7 → n=8) would require an origin of new centromere by an unknown mechanism. Although the emergence of de novo centromeres (Nasuda et al., 2005) and centric fissions (Jones, 1998) is documented in plants, these have not been observed in crucifers as yet.
As ancestral chromosomes per se do not provide insight into the evolutionary relationship among the analyzed species, intertribal relationships have been elucidated through evolutionarily novel associations of crucifer GBs. The chromosome AK6/8 (association of O/P/W/R blocks) and the collinearity of V/K/L/Q/X blocks found in all x=7 species serve as unique cytogenetic signatures unambiguously underlying the monophyletic origin of all six tribes (Figure 4). Chromosome AK6/8 and the association of V/K/L/Q/X blocks must have been present already in the PCK. Eutremeae, Isatideae, and Sisymbrieae share a younger reciprocal whole-arm translocation between chromosomes AK5/8/6 and AK2 (Figure 4).
Cytogenetic Signatures: Phylogenetic Implications
Cytogenetic signatures (e.g., novel associations of GBs) can be used to delimit taxa, as chromosome rearrangements generally exhibit only a low level of homoplasy and thus have the power to disentangle unresolved or conflicting phylogenetic relationships. The tribe Eutremeae (∼25 species) had an unresolved position within the group of x=7 tribes (Figure 1B) (Beilstein et al., 2006; Koch et al., 2007; Koch and Al-Shehbaz, 2008). We have shown that the karyotype of Eutremeae (T. halophila) is identical to that of Isatideae and Sisymbrieae but different from that of Calepineae, Conringieae, and Noccaeeae. Hence, this supports the inclusion of Eutremeae together with Brassiceae, Isatideae, Schizopetaleae, and Sisymbrieae in Lineage II, forming a monophyletic group (Figure 4). The data here corroborate the earlier exclusion of Calepineae and Conringieae from the Brassiceae (Lysak et al., 2005). Although Calepineae and Conringieae possess similar karyotypes, differences in morphological characters substantiate their recent circumscription as two closely related tribes (German and Al-Shehbaz, 2008). CCP data are also congruent with the close relationship between Conringieae and the tribe Noccaeeae revealed by nuclear and chloroplast gene phylogenies (Bailey et al., 2006; Beilstein et al., 2006; German and Al-Shehbaz, 2008). The reconstructed chromosomal phylogeny elucidated relationships among the analyzed x=7 tribes and provided compelling evidence that tribes of Lineage II (including Eutremeae) and Calepineae, Conringieae, and Noccaeeae have a monophyletic origin (Figure 4). Further CCP analysis of other tribes currently placed into the phylogenetic proximity of Lineage II (Figure 1B) is needed to further clarify phylogenetic relationships and genome evolution within this crucifer group.
By analyzing species with an identical chromosome number (2n=14 or 28) from six closely related but distinct tribes, we also tested for the functional role of gross karyotypic alterations during speciation. Our data indicate that even morphologically distinct groups such as Calepineae/Conringieae/Noccaeeae, when compared with tribes of Lineage II, display almost complete genome collinearity and very similar karyotypes. It remains elusive to what extent the Lineage II–specific translocation resulting in chromosomes AK2/5 and AK2/5/6/8 contributed to the divergence of Lineage II tribes. However, the resolution of CCP analysis is constrained by the size of BAC painting probes, reducing the ability to detect minor rearrangements that might be of evolutionary significance. The above findings indicate that the speciation resulting in morphologically distinct but closely related taxa (Al-Shehbaz et al., 2006; German and Al-Shehbaz, 2008) is not necessarily accompanied or determined by major karyotypic changes.
Karyotype Evolution Preceding the Whole-Genome Triplication in Brassiceae
As Isatideae, Sisymbrieae, and Eutremeae along with tribes Brassiceae and Schizopetaleae form a monophyletic group called Lineage II (Beilstein et al., 2006; Koch et al., 2007; this study), some conclusions on the early evolution of the whole lineage can be drawn from the data presented here. The chromosome homeology patterns of Isatideae, Sisymbrieae, and Eutremeae can be compared with the structure of Brassica genomes (tribe Brassiceae) revealed by comparative genetic analysis (Parkin et al., 2005). This comparison showed that the R/W block association (=long arm of AK6/8) as well as the V/K/L/Q/X association are present also in the Brassica rapa component (n=10) of the B. napus genome (n=19) (Figure 4) (Parkin et al., 2005; Schranz et al., 2006). Three copies of the R/W block association are located on B. napus chromosomes N2, N3, and N10, whereas the V/K/L/Q/X association has been found in two copies on chromosomes N2 and N6 (only blocks Q and V of the third genomic copy were detected on chromosome N9 [Figure 4]). Due to the whole-genome triplication in the tribe Brassiceae (Lysak et al., 2005; Parkin et al., 2005), three instead of two copies of the V/K/L/Q/X association are to be expected. Nevertheless, this pattern corresponds well with the fragmentary character of Brassica genomes, as the genomic redundancy after the whole-genome triplication was followed by the diploidization eroding the original triplicated pattern. Consequently, some GBs were retained in three copies, other GBs as well as smaller segments were preserved as one or two copies, and some were secondarily multiplied, probably through unequal homeologous recombination (Lukens et al., 2004; Parkin et al., 2005). The present structure of the B. napus genome does not allow us to arrive at a clear-cut conclusion whether the AK5/6/8–AK2 translocation is shared by all tribes of Lineage II or whether it is specific only for Isatideae, Sisymbrieae, and Eutremeae. Regardless of this limitation, the aforementioned cytogenetic signatures provide convincing evidence that Brassiceae and the whole Lineage II descended from the PCK (n=7). In the clade leading to Brassiceae, the PCK karyotype has undergone the whole-genome triplication. This parsimonious scenario does not rule out the possibility that the tribe-specific triplication was preceded by another round of chromosome number reduction (n=7 → n=6), as x=6 was suggested as an ancestral base chromosome number of Brassica species by several authors (Catcheside, 1937; Röbbelen, 1960). However, the present data do not warrant any conclusion regarding the chromosome number of the primary hexaploid (Lysak et al., 2007).
Mechanism of Chromosome Number Reduction from n=8 to n=7
Chromosome rearrangements revealed in the x=7 taxa can be reconstructed by considering only inversions and reciprocal translocations. Chromosome number reduction from n=8 to n=7 implies the loss or inactivation of one centromere. Our data indicate that the centromere loss occurred as a result of a reciprocal translocation between an acrocentric chromosome (formed via a preceding pericentric inversion) and a (sub)metacentric chromosome. This scenario yields a translocation chromosome (AK5/6/8) and a minichromosome comprising mainly the centromere of the acrocentric chromosome (Figure 2E). The meiotically unstable minichromosome is eliminated. The same mechanism has been argued as responsible for chromosome number reduction in tribes of Lineage I (Lysak et al., 2006) and is assumed to be a prominent mechanism reducing chromosome numbers in Brassicaceae (Schranz et al., 2006; Schubert, 2007). Other chromosome rearrangements identified in the x=7 species included pericentric and paracentric inversions and whole-arm reciprocal translocations. No evidence for duplications or deletions of GBs has yet been detected.
We have scrutinized the number of rearrangement events and corresponding break points necessary to explain the structure of the extant x=7 karyotypes. The origin of the PCK (i.e., translocation chromosomes AK6/8 and AK5/6/8) from an ancestral n=8 karyotype requires five events and nine break points (five in centromeric, two in terminal, and two in interstitial regions at W/X and Q/R boundaries). The reciprocal translocation between chromosomes AK2 and AK5/6/8 yielding the Lineage II–specific chromosomes AK2/5 and AK2/5/6/8 involves another two pericentromeric break points. In G. laevigata, six species-specific break points have been identified (one pericentromeric and five interstitial, including one coinciding with the O/P boundary). In Glastaria, 11 plus 2 species-specific break points at K/L and Q/X boundaries were identified. The highest number of break points is in N. caerulescens, with 9 break points shared with the PCK and 20 additional break points necessary to account for species-specific pericentric and paracentric inversions (Figure 2D; see Supplemental Figure 2 online). Of the 20 break points, 10 map to pericentromeric regions, whereas 10 are at interstitial positions (two at T/U and W/R boundaries). In total, of the 11 basic break points necessary for the origin of the three translocation chromosomes, 9 map to pericentromeric or telomeric regions and only 2 are interstitial. From a total of 28 secondary species-specific break points, 11 localize to centromeres. Thus, from all 39 break points, half (20 break points) involve telomeric/pericentromeric regions and the other half (19 break points) occur at interstitial positions. Although pericentromeric and telomeric regions characterized by clusters of tandem repeats are considered as chromosome regions prone to chromosome rearrangements, most likely by etopic (nonallelic) homologous recombination (Gaut et al., 2007; Schubert, 2007), the observed equal frequency of interstitial break points suggests that large clusters of repetitive sequence elements are not the exclusive sites of chromosomal breakage. Segmental duplications were proposed as regions facilitating chromosome rearrangements, such as inversions in primate genomes (Kehrer-Sawatzki and Cooper, 2008). In Drosophila, duplications of nonrepetitive sequences at the break point regions of ∼60% of the analyzed inversions were presumably caused by staggered breaks rather than by ectopic recombination between repetitive elements (Ranz et al., 2007). Future genome sequencing of the x=7 species (e.g., N. caerulescens and T. halophila) will unveil the detailed sequence structure of the break points.
Diverse Routes of Chromosome Number Reduction from n=8 to n=7
So far, data on karyotype evolution in Brassicaceae suggest that at least two major phylogenetic branches (Lineage I and II) descended from a common ancestral karyotype with eight chromosome pairs. We assume that in some crucifer taxa, the number of ancestral linkage groups was reduced toward karyotypes with n=5 to n=7. One crucial question is whether chromosome number reduction within diverse phylogenetic lineages is based on the same or on different events. Among crucifer x=7 taxa, the genome structure of only two species from Lineage I, Neslia paniculata and Boechera stricta, was analyzed with a sufficient precision (Lysak et al., 2006; Schranz et al., 2007). In N. paniculata (Camelineae), the reduction combined the ancestral chromosomes AK4 and AK5. Both chromosomes became acrocentric via pericentric and paracentric inversions and fused by a translocation with break points close to the centromeres. The chromosome AK4/5 comprises blocks I/J (from AK4) on the short arm and the K/L/M/N block association (from AK5) on its long arm (Lysak et al., 2006). In B. stricta (Boechereae), chromosome number reduction followed a more complex scenario than in Neslia (Schranz et al., 2007). The reduction involved translocations between five AK chromosomes and resulted in translocation chromosomes AK1/2 (blocks A/B/C/D), AK3/8 (blocks F/G/W/X), and AK3/5/8 (blocks K/L/M/N/V/H). In Neslia and Boechera (Lineage I) as well as in the x=7 tribes affiliated with Lineage II, the ancestral chromosome AK5 is always involved in chromosome number reduction; however, the changes leading to the reduction differ. In Neslia and Boechera, all AK5-derived GBs (K/L/M/N) remain associated within new chromosomes, whereas in the x=7 species analyzed here, the GBs are split up within AK5/6/8 or between chromosomes AK2/5 and AK2/5/6/8 (Figure 4). The comparison here clearly shows that chromosome number reduction in Lineage I (Camelineae and Boechereae) is different from that of the x=7 tribes associated with Lineage II. The repeated participation of certain ancestral chromosomes in diverse rearrangements was also observed in other groups such as grasses (Srinivasachary et al., 2007).
Heterochromatic Landmarks in the x=7 Species
The analyzed x=7 species show a rather uniform pattern of rDNA loci and heterochromatic knobs. In our analyzed x=7 species, all NORs were located interstitially, adjacent to pericentromeric heterochromatic regions. This is in contrast with the prevalence of terminal NORs found elsewhere in the family (Ali et al., 2005), including Camelineae and Descurainieae species analyzed by CCP (Berr et al., 2006; Lysak et al., 2006).
The number of 5S rDNA loci was two or four, except in O. aegyptiacum, with 14 loci (Table 1; see Supplemental Figure 3 online). The remarkable localization of 5S rDNA on all chromosomes of a complement is not yet known in higher plants (Cloix et al., 2003). This high number of heterochromatic 5S rDNA sites is most likely responsible for the severe stickiness of prophase I bivalents at pericentromeric heterochromatic regions in this species. Similarly, better spread pachytene bivalents were obtained in A. thaliana accessions with only two major 5S rDNA loci compared with the accessions having 5S rDNA on three chromosomes (Fransz et al., 1998). It remains unclear whether the chromosomal ubiquity of 5S rDNA in O. aegyptiacum was caused by the mobility of transposable elements, similar to the En/Spm transposon–mediated transfer of 5S rDNA observed in Aegilops (Raskina et al., 2004, 2008).
The frequent mobility of 45S and 5S rDNA sites (Pontes et al., 2004; Schubert, 2007; Raskina et al., 2008) precludes any plausible assumption on the ancestral distribution of rDNA sites. While the loss of some NORs can occur during chromosome number reduction events (Lysak et al., 2006; Schranz et al., 2006; Schubert, 2007) and other rDNA clusters can be amplified or transposed via ectopic recombination or due to the activity of transposable elements (Pontes et al., 2004; Raskina et al., 2004, 2008), direct evidence for the involvement of rDNA repeats in crucifer chromosome rearrangements is lacking.
Three x=7 species possess heterochromatic knobs positioned terminally or interstitially. In plants, knobs can originate through inversions, including pericentromeric heterochromatin (e.g., the hk4S knob in A. thaliana [Fransz et al., 2000]), the insertion and subsequent silencing of repetitive elements such as tandem repetitive transgenes (Probst et al., 2003), or can be copied from other chromosomal regions due to the physical proximity within interphase nuclei (Zhong et al., 1998). Apart from the hk4S knob in A. thaliana (Fransz et al., 2000) and small interstitial as well as distal knobs in Brassica species (Röbbelen, 1960; Lim et al., 2005; Lysak et al., 2007), large terminal heterochromatic knobs are not yet reported in Brassicaceae species. These terminal knobs resemble the knobs on chromosomes of maize (Zea mays; Lamb et al., 2007), rice (Oryza sativa; Ohmido et al., 2001), or tomato (Solanum lycopersicum; Zhong et al., 1998). As in these species, it is likely that knobs in crucifer species will primarily comprise tandem repeats. Satellite repeats composing distal knobs in the above species were classified as subtelomeric with a potential functional role (Lamb et al., 2007). Three large interstitial knobs within the T. halophila karyotype (Figure 3E) are not linked to a higher frequency of species-specific chromosome rearrangements, as seen in N. caerulescens, a species with only a single knob (Figure 2D). Our data suggest that interstitial as well as terminal knobs, revealed only in C. orientalis (Figure 2A) and M. perfoliatum (Figure 3A), are apparently of more recent origin and did not mediate the described chromosome repatterning.
Cytogenetic Evidence of Genome Diploidization in Autotetraploid Calepineae Species
In this study, the complete karyotype structure of two tetraploid Calepineae species was analyzed. In C. irregularis, diploid (2n=2x=14) and tetraploid (2n=4x=28) cytotypes are known, whereas only tetraploids are recorded for G. laevigata (Warwick and Al-Shehbaz, 2006). As Calepina is a monospecific genus (German and Al-Shehbaz, 2008), we assume that the 4x genome was derived from the 2x cytotype via autopolyploidy relatively recently. Indeed, our CCP data corroborated the autopolyploid origin of the 4x cytotype, as all seven chromosomes showed the same structure in both genomes (Figure 2B). In G. laevigata, one pair each of AK1, AK7, and AK6/8 differs from the other pair by pericentric inversions (Figure 2C). Although an allopolyploid origin of the tetraploid G. laevigata cannot be ruled out within the genus comprising five other species (German and Al-Shehbaz, 2008), autopolyploidization followed by three species-specific inversions is a more likely interpretation. No records of a diploid cytotype may indicate an earlier origin of the tetraploid G. laevigata compared with the 4x cytotype of C. irregularis. This is underlined by the pericentric inversions that differentiate three of seven homologous pairs of G. laevigata. Although chromosome rearrangements may reduce the risk of meiotic multivalent formation and thus contribute to the genome diploidization of autopolyploids (Ma and Gustafson, 2005), regular meiotic pairing and 14 bivalents have been observed in both species (see Supplemental Figure 1 online). This implies that the diploid-like meiosis in the two autopolyploids is determined genetically rather than by gross intrachromosomal rearrangements. A partial diploidization of meiosis was also observed in established autotetraploid lines of A. thaliana (Santos et al., 2003).
METHODS
Plant Material
All but one species were grown from seeds obtained from the Millennium Seed Bank, Royal Botanic Gardens, Kew (Glastaria glastifolia, Ochthodium aegyptiacum), the Botanical Garden of Bordeaux (Calepina irregularis, Conringia orientalis), the Hortus Botanicus Hauniensis, Copenhagen (Goldbachia laevigata, Myagrum perfoliatum), and from D. Baum, University of Wisconsin, Madison (Thellungiella halophila). Inflorescences of Noccaea caerulescens were collected from a single wild population (Kořenec, Czech Republic). Plants grown from seeds were cultivated in a greenhouse or growth chamber under long-day light conditions. Herbarium vouchers of all analyzed species are deposited in the herbarium of Masaryk University.
Preparation of Pachytene Chromosomes
Entire inflorescences were fixed in ethanol:chloroform:acetic acid (6:3:1) overnight and stored in 70% ethanol at −20°C until use. Selected flower buds were rinsed in distilled water (2 × 5 min) and in citrate buffer (10 mM sodium citrate, pH 4.8; 2 × 5 min) and incubated in an enzyme mix (0.3% cellulase, cytohelicase, and pectolyase; all Sigma-Aldrich) in citrate buffer at 37°C for 3 to 6 h, transferred into citrate buffer, and kept at 4°C until use (overnight to 2 d). Individual anthers were put on a microscope slide of a stereomicroscope and disintegrated by a needle in a drop of citrate buffer. Then the suspension was softened by adding 15 to 30 μL of 60% acetic acid and spread by stirring with a needle on a hot plate at 50°C for 0.5 to 2 min. Chromosomes were fixed by adding 100 μL of ethanol:acetic acid (3:1) fixative. The slide was tilted to remove the fixative and dried with a hair dryer. The dried preparation was staged with a phase contrast microscope. Suitable slides were postfixed in 4% formaldehyde dissolved in distilled water for 10 min and air-dried.
Painting Probes
Arabidopsis thaliana BAC clones were obtained from the ABRC. The selection of clones suitable for chromosome painting and DNA isolation from individual BAC clones was performed as described by Lysak et al. (2003). For CCP, each third BAC was used and contigs were arranged and differentially labeled according to the GBs of the ACK (Schranz et al., 2006). For BAC contigs used for CCP, see Supplemental Table 1 online. To characterize species-specific inversions in N. caerulescens and G. laevigata, the respective BAC contigs were split into smaller subcontigs and used as CCP probes. The BAC clone T15P10 (AF167571) bearing 45S rRNA genes was used for in situ localization of NORs, and clone pCT 4.2, corresponding to a 500-bp 5S rRNA repeat (M65137), was used for localization of 5S rDNA loci.
All DNA probes were labeled by nick translation with biotin-dUTP, digoxigenin-dUTP, or Cy3-dUTP as follows: 1 μg of DNA diluted in distilled water to 29 μL, 5 μL of nucleotide mix (2 mM dATP, dCTP, and dGTP, 400 μM dTTP; all Roche), 5 μL of 10× NT buffer (0.5 M Tris-HCl, pH 7.5, 50 mM MgCl2, and 0.05% BSA), 4 μL of 1 mM X-dUTP (in which X was biotin, digoxigenin, or Cy3), 5 μL of 0.1 M β-mercaptoethanol, 1 μL of DNase I (Roche), and 1 μL of DNA polymerase I (Fermentas). The nick translation mixture was incubated at 15°C for 90 min (or longer) to obtain a fragment length of ∼200 to 500 bp. The nick translation reaction was stopped by adding 1 μL of 0.5 M EDTA, pH 8.0, and incubation at 65°C for 10 min. Individual labeled probes were stored at −20°C until use.
CCP
To remove cytoplasm, the slides were treated with pepsin (0.1 μg/μL; Sigma-Aldrich) in 0.01 M HCl for 3 to 6 h, postfixed in 4% formaldehyde dissolved in 2× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) for 10 min, dehydrated in an ethanol series (70, 80, and 96%), and air-dried.
Selected BAC clones were pooled and precipitated by adding one-tenth volume of 3 M sodium acetate, pH 5.2, and 2.5 volumes of ice-cold 96% ethanol, kept at −20°C for 30 min, and centrifuged at 13,000g at 4°C for 30 min. The pellet was resuspended in 20 μL of hybridization mix (50% formamide and 10% dextran sulfate in 2× SSC) per slide. Cover slips were framed by rubber cement. The probe and chromosomes were denatured together on a hot plate at 80°C for 2 min and incubated in a moist chamber at 37°C for 39 to 63 h.
Posthybridization washing was performed in 50% formamide (A. thaliana, which was used as a probe quality control) or 20% formamide (all other species) in 2× SSC at 42°C. Fluorescent detection was as follows: biotin-dUTP was detected by avidin–Texas Red (Vector Laboratories) and amplified by goat anti-avidin–biotin (Vector Laboratories) and avidin–Texas Red; digoxigenin-dUTP was detected by mouse anti-digoxigenin (Jackson ImmunoResearch) and goat anti-mouse Alexa Fluor 488 (Molecular Probes), and Cy3-dUTP labeled probes were observed directly. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (2 μg/mL) in Vectashield (Vector Laboratories).
Fluorescence signals were analyzed with an Olympus BX-61 epifluorescence microscope and AxioCam CCD camera (Zeiss). Images were acquired separately for all four fluorochromes using appropriate excitation and emission filters (AHF Analysentechnik). The four monochromatic images were pseudocolored and merged using Adobe Photoshop CS2 software (Adobe Systems).
Chromosome Measurements and Genome Size Estimation
The length of painted GBs was measured using the ImageJ program (National Institutes of Health). The nuclear DNA content has been estimated by flow cytometry at the Institute of Botany and Zoology, Masaryk University. For details, see Lysak et al. (2007).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Examples of CCP in Tetraploid Calepineae Species (2n=4x=28).
Supplemental Figure 2. Species-Specific Chromosome Rearrangements (Inversion Events) in N. caerulescens.
Supplemental Figure 3. Heterochromatic Landmarks in O. aegyptiacum and T. halophila.
Supplemental Table 1. GBs of the ACK and Corresponding A. thaliana BAC Contigs Used as Painting Probes in This Study.
Supplementary Material
Acknowledgments
We thank Petr Bureš for genome size estimates and Ingo Schubert, Chris Pires, and M. Eric Schranz for their valuable comments on the manuscript. Dmitry German, Marcus Koch, and Klaus Mummenhoff are acknowledged for providing unpublished phylogenetic data. This study was supported by research grants from the Grant Agency of the Czech Academy of Science (Grant KJB601630606) and the Czech Ministry of Education (Grant MSM0021622415).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Martin A. Lysak (lysak@sci.muni.cz).
Online version contains Web-only data.
References
- Ali, H.B.M., Lysak, M.A., and Schubert, I. (2005). Chromosomal localization of rDNA in the Brassicaceae. Genome 48 341–346. [DOI] [PubMed] [Google Scholar]
- Al-Shehbaz, I.A., Beilstein, M.A., and Kellogg, E.A. (2006). Systematics and phylogeny of the Brassicaceae (Cruciferae): An overview. Plant Syst. Evol. 259 89–120. [Google Scholar]
- Bailey, C.D., Koch, M.A., Mayer, M., Mummenhoff, K., O'Kane, S.L., Jr., Warwick, S.I., Windham, M.D., and Al-Shehbaz, I.A. (2006). Toward a global phylogeny of the Brassicaceae. Mol. Biol. Evol. 23 2142–2160. [DOI] [PubMed] [Google Scholar]
- Beilstein, M.A., Al-Shehbaz, I.A., and Kellogg, E.A. (2006). Brassicaceae phylogeny and trichome evolution. Am. J. Bot. 93 607–619. [DOI] [PubMed] [Google Scholar]
- Berr, A., Pecinka, A., Meister, A., Kreth, G., Fuchs, J., Blattner, F.R., Lysak, M.A., and Schubert, I. (2006). Chromosome arrangement and nuclear architecture but not centromeric sequences are conserved between Arabidopsis thaliana and Arabidopsis lyrata. Plant J. 48 771–783. [DOI] [PubMed] [Google Scholar]
- Boivin, K., Acarkan, A., Mbulu, R.S., Clarenz, O., and Schmidt, R. (2004). The Arabidopsis genome sequence as a tool for genome analysis in Brassicaceae: A comparison of the Arabidopsis and Capsella rubella genomes. Plant Physiol. 135 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catcheside, D.G. (1937). Secondary pairing in Brassica oleracea. Cytologia Fujii Jub. 366–378.
- Cloix, C., Tutois, S., and Tourmente, S. (2003). 5S rDNA and 5S RNA in higher plants. Rec. Res. Dev. Plant Mol. Biol. 1 207–221. [Google Scholar]
- Devos, K.M. (2005). Updating the ‘crop circle.’ Curr. Opin. Plant Biol. 8 155–162. [DOI] [PubMed] [Google Scholar]
- Fransz, P., Armstrong, S., Alonso-Blanco, C., Fischer, T.C., Torres-Ruiz, R.A., and Jones, G.H. (1998). Cytogenetics for the model system Arabidopsis thaliana. Plant J. 13 867–876. [DOI] [PubMed] [Google Scholar]
- Fransz, P.F., Armstrong, S., de Jong, J.H., Parnell, L.D., van Drunen, G., Dean, C., Zabel, P., Bisseling, T., and Jones, G.H. (2000). Integrated cytogenetic map of chromosome arm 4S of A. thaliana: Structural organization of heterochromatic knob and centromere region. Cell 100 367–376. [DOI] [PubMed] [Google Scholar]
- Gaut, B.S., Wright, S.I., Rizzon, C., Dvorak, J., and Anderson, L.K. (2007). Recombination: An underappreciated factor in the evolution of plant genomes. Nat. Rev. Genet. 8 77–84. [DOI] [PubMed] [Google Scholar]
- German, D.A., and Al-Shehbaz, I.A. (2008). Five additional tribes (Aphragmeae, Biscutelleae, Calepineae, Conringieae, and Erysimeae) in the Brassicaceae (Cruciferae). Harv. Pap. Bot. 13 165–170. [Google Scholar]
- Gong, Q., Li, P.H., Ma, S.S., Rupassara, S.I., and Bohnert, H.J. (2005). Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 44 826–839. [DOI] [PubMed] [Google Scholar]
- Jiang, J., and Gill, B.S. (2006). Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 49 1057–1068. [DOI] [PubMed] [Google Scholar]
- Jones, K. (1998). Robertsonian fusion and centric fission in karyotype evolution of higher plants. Bot. Rev. 64 273–289. [Google Scholar]
- Kehrer-Sawatzki, H., and Cooper, D.N. (2008). Molecular mechanisms of chromosomal rearrangement during primate evolution. Chromosome Res. 16 41–56. [DOI] [PubMed] [Google Scholar]
- Koch, M., and Al-Shehbaz, I.A. (2008). Molecular systematics and evolution of “wild” crucifers (Brassicaceae or Cruciferae). In Biology and Breeding of Crucifers, P.K. Gupta, ed (London: Taylor and Francis Group).
- Koch, M.A., Dobeš, C., Kiefer, C., Schmickl, R., Klimes, L., and Lysak, M.A. (2007). Supernetwork identifies multiple events of plastid trnF(GAA) pseudogene evolution in the Brassicaceae. Mol. Biol. Evol. 24 63–73. [DOI] [PubMed] [Google Scholar]
- Koch, M.A., and Kiefer, M. (2005). Genome evolution among cruciferous plants: A lecture from the comparison of the genetic maps of three diploid species—Capsella rubella, Arabidopsis lyrata subsp. petraea, and A. thaliana. Am. J. Bot. 92 761–767. [DOI] [PubMed] [Google Scholar]
- Kuittinen, H., de Haan, A.A., Vogl, C., Oikarinen, S., Leppala, J., Koch, M., Mitchell-Olds, T., Langley, C.H., and Savolainen, O. (2004). Comparing the linkage maps of the close relatives Arabidopsis lyrata and A. thaliana. Genetics 168 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, C.J., Meyer, M.J., Corcoran, B., Kato, A., Han, F., and Birchler, A.J. (2007). Distinct chromosomal distributions of highly repetitive sequences in maize. Chromosome Res. 15 33–49. [DOI] [PubMed] [Google Scholar]
- Lim, K.B., et al. (2005). Characterization of rDNAs and tandem repeats in the heterochromatin of Brassica rapa. Mol. Cells 19 436–444. [PubMed] [Google Scholar]
- Lukens, L.N., Quijada, P.A., Udall, J., Pires, J.C., Schranz, M.E., and Osborn, T.C. (2004). Genome redundancy and plasticity within ancient and recent Brassica crop species. Biol. J. Linn. Soc. 82 665–674. [Google Scholar]
- Lysak, M., Berr, A., Pecinka, A., Schmidt, R., McBreen, K., and Schubert, I. (2006). Mechanisms of chromosome number reduction in Arabidopsis thaliana and related Brassicaceae species. Proc. Natl. Acad. Sci. USA 103 5224–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysak, M.A., Cheung, K., Kitschke, M., and Bureš, P. (2007). Ancestral chromosomal blocks are triplicated in Brassiceae species with varying chromosome number and genome size. Plant Physiol. 145 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysak, M.A., Koch, M.A., Pecinka, A., and Schubert, I. (2005). Chromosome triplication found across the tribe Brassiceae. Genome Res. 15 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysak, M.A., and Lexer, C. (2006). Towards the era of comparative evolutionary genomics in Brassicaceae. Plant Syst. Evol. 259 175–198. [Google Scholar]
- Lysak, M.A., Pecinka, A., and Schubert, I. (2003). Recent progress in chromosome painting of Arabidopsis and related species. Chromosome Res. 11 195–204. [DOI] [PubMed] [Google Scholar]
- Ma, X.F., and Gustafson, J.P. (2005). Genome evolution of allopolyploids: A process of cytological and genetic diploidization. Cytogenet. Genome Res. 109 236–249. [DOI] [PubMed] [Google Scholar]
- Nasuda, S., Hudakova, S., Schubert, I., Houben, A., and Endo, T.R. (2005). Stable barley chromosomes without centromeric repeats. Proc. Natl. Acad. Sci. USA 102 9842–9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmido, N., Kijima, K., Ashikawa, I., de Jong, J.H., and Fukui, K. (2001). Visualization of the terminal structure of rice chromosomes 6 and 12 with multicolor FISH to chromosomes and extended DNA fibers. Plant Mol. Biol. 47 413–421. [DOI] [PubMed] [Google Scholar]
- Parkin, I.A.P., Gulden, S.M., Sharpe, A.G., Lukens, L., Trick, M., Osborn, T.C., and Lydiate, D.J. (2005). Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171 765–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer, W.A., Mahmoudian, M., Freeman, J.L., Lahner, B., Richards, E.L., Reeves, R.D., Murphy, A.S., and Salt, D.E. (2006). Assessment of plants from the Brassicaceae family as genetic models for the study of nickel and zinc hyperaccumulation. New Phytol 172 248–260. [DOI] [PubMed] [Google Scholar]
- Pontes, O., Neves, N., Silva, M., Lewis, M.S., Madlung, A., Comai, L., Viegas, W., and Pikaard, C.S. (2004). Chromosomal locus rearrangements are a rapid response to formation of the allotetraploid Arabidopsis suecica genome. Proc. Natl. Acad. Sci. USA 101 18240–18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst, A.V., Fransz, P.F., Paszkowki, J., and Mittelsten Scheid, O. (2003). Two means of transcriptional reactivation within heterochromatin. Plant J. 33 743–749. [DOI] [PubMed] [Google Scholar]
- Ranz, J.M., Maurin, D., Chan, Y.S., von Grotthuss, M., Hillier, L.W., Roote, J., Ashburner, M., and Bergman, C.M. (2007). Principles of genome evolution in the Drosophila melanogaster species group. PLoS Biol. 5 1366–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskina, O., Barber, J.C., Nevo, E., and Belyayev, A. (2008). Repetitive DNA and chromosomal rearrangements: Speciation-related events in plant genomes. Cytogenet. Genome Res. 120 351–357. [DOI] [PubMed] [Google Scholar]
- Raskina, O., Belyayev, A., and Nevo, E. (2004). Quantum speciation in Aegilops: Molecular cytogenetic evidence from rDNA cluster variability in natural populations. Proc. Natl. Acad. Sci. USA 101 14818–14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röbbelen, G. (1960). Beiträge zur Analyse des Brassica-Genoms. Chromosoma 11 205–228. [DOI] [PubMed] [Google Scholar]
- Santos, J.L., Alfaro, D., Sanchez-Moran, E., Armstrong, S.J., Franklin, F.C.H., and Jones, G.H. (2003). Partial diploidization of meiosis in autotetraploid Arabidopsis thaliana. Genetics 165 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz, M.E., Lysak, M.A., and Mitchell-Olds, T. (2006). The ABC's of comparative genomics in the Brassicaceae: Building blocks of crucifer genomes. Trends Plant Sci. 11 535–542. [DOI] [PubMed] [Google Scholar]
- Schranz, M.E., Windsor, A.J., Song, B., Lawton-Rauh, A., and Mitchell-Olds, T. (2007). Comparative genetic mapping in Boechera stricta, a close relative of Arabidopsis. Plant Physiol. 144 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, I. (2007). Chromosome evolution. Curr. Opin. Plant Biol. 10 109–115. [DOI] [PubMed] [Google Scholar]
- Schubert, I., Fransz, P.F., Fuchs, J., and de Jong, J.H. (2001). Chromosome painting in plants. Methods Cell Sci. 23 57–69. [PubMed] [Google Scholar]
- Srinivasachary, Dida, M.M., Gale, M.D., and Devos, K.M. (2007). Comparative analyses reveal high levels of conserved colinearity between the finger millet and rice genomes. Theor. Appl. Genet. 115 489–499. [DOI] [PubMed] [Google Scholar]
- Warwick, S.I., and Al-Shehbaz, I.A. (2006). Brassicaceae: Chromosome number index and database on CD-ROM. Plant Syst. Evol. 259 237–248. [Google Scholar]
- Wei, F., et al. (2007). Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genet. 3 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, X.B., Fransz, P.F., Wennekes-van Eden, J., Ramanna, M.S., van Kammen, A., Zabel, P., and de Jong, J.H. (1998). FISH studies reveal the molecular and chromosomal organization of individual telomere domains in tomato. Plant J. 13 507–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.