Abstract
NMDA-type glutamate receptors (NMDARs) mediate many forms of synaptic plasticity. These tetrameric receptors consist of two obligatory NR1 subunits and two regulatory subunits, usually a combination of NR2A and NR2B. In the neonatal neocortex NR2B-containing NMDARs predominate, and sensory experience facilitates a developmental switch in which NR2A levels increase relative to NR2B. In this review, we clarify the roles of NR2 subunits in synaptic plasticity, and argue that a primary role of this shift is to control the threshold, rather than determining the direction, for modifying synaptic strength. We also discuss recent studies that illuminate the mechanisms regulating NR2 subunits, and suggest that the NR2A/NR2B ratio is regulated by multiple means, which may control the ratio both locally at individual synapses and globally in a cell-wide manner. Finally, we use the visual cortex as a model system to illustrate how activity-dependent modifications in the NR2A/NR2B ratio may contribute to the development of cortical functions.
1. Introduction
NMDARs and AMPA-type glutamate receptors (AMPARs) are key mediators of excitatory synaptic transmission in the brain. Both receptor types are glutamate-gated cation channels that convert a chemical signal (glutamate released from presynaptic terminals) to an electric signal (a membrane voltage change due to cation flow though the channels). Most NMDAR subtypes are unique in that their opening requires the coincidence of both presynaptic glutamate release and a strong postsynaptic membrane depolarization to relieve Mg2+ block of the channel (Mayer et al., 1984; Nowak et al., 1984). NMDARs are permeable to Na+, K+, and Ca2+ ions, the latter of which acts as a second messenger to modify synapses (Lynch et al., 1983). These receptor properties promote input specificity of Ca2+-dependent synaptic modifications by NMDARs.
Two paradigmatic examples of changes in synaptic strength are long-term depression (LTD) and long-term potentiation (LTP), which are induced in a variety of brain regions with diverse stimulation protocols (Malenka and Bear, 2004). Many forms of LTD and LTP require NMDAR activation and the subsequent cascade of events triggered by Ca2+ influx. Those events include AMPAR removal from (LTD), or insertion into (LTP), postsynaptic membranes (Malenka and Bear, 2004) and changes in spine morphology (Matsuzaki et al., 2004; Zhou et al., 2004). The direction of the plasticity (weakening or strengthening) is controlled largely by the kinetics and amount of Ca2+ influx through NMDARs. In the case of frequency-dependent forms of synaptic plasticity, the magnitude and time course of Ca2+ entry is determined by the frequency of the conditioning stimulations given to axonal fibers. For example, 100 Hz stimulation of axonal fibers for 1-3 seconds induces rapid and robust Ca2+ entry through NMDARs, resulting in LTP (Bliss and Lomo, 1973; Malenka and Bear, 2004). Alternatively, 0.5-5 Hz stimulation lasting for 5-30 minutes allows a smaller magnitude of Ca2+ entry through NMDARs over a longer time course, leading to LTD (Dudek and Bear, 1992; Malenka and Bear, 2004; Yang et al., 1999). The level of Ca2+ influx through NMDARs is determined in part by the level of postsynaptic membrane depolarization, as this determines the extent that NMDARs are relieved from Mg2+ block. Thus, even with low frequency stimulation, LTP can be induced if the postsynaptic cells are held at depolarized membrane potentials (Kelso et al., 1986; Wigstrom and Gustafsson, 1986).
NMDARs are thought to consist of four subunits: two obligatory NR1 subunits and two regulatory subunits that can be NR2A→D, or NR3A→B. The precise combination of NMDAR subunits determines the functional properties of the NMDAR channels (Cull-Candy and Leszkiewicz, 2004). Additional heterogeneity of NMDAR functions can arise through alternative splicing (reviewed in Cull-Candy et al., 2001). Both the NMDAR subunit composition (Chen et al., 2000; Liu et al., 2004b; Nase et al., 1999; Quinlan et al., 1999a; Roberts and Ramoa, 1999) and the alternative splicing of NR1 subunits (Laurie and Seeburg, 1994; Prybylowski and Wolfe, 2000) change during development. NR2A and NR2B subunits, which predominate NMDAR subtypes in the forebrain, undergo a particularly well-characterized developmental shift in the cortex. NR2B subunits are abundant in the early postnatal brain, and NR2A levels increase progressively with development (Flint et al., 1997; Mierau et al., 2004; Quinlan et al., 1999a; Roberts and Ramoa, 1999; Sheng et al., 1994). Sensory deprivation retards the NR2B to NR2A shift in NMDAR composition (Liu et al., 2004b; Nase et al., 1999; Quinlan et al., 1999a; Roberts and Ramoa, 1999), suggesting this subunit change is guided in part by sensory experience.
In this review, we will discuss three fundamental questions regarding NR2A and NR2B subunits. First, what are the molecular bases for the activity-dependent regulation of the NR2A/NR2B ratio? Second, what are the roles of NR2A and NR2B in LTD and LTP? Third, what is the functional consequence of the NR2A/NR2B ratio change in synaptic plasticity in vitro and in vivo? For the latter issue, we focus on the visual cortex as a model system. This review is not meant to be comprehensive, but rather is meant to highlight recent literature, to address controversies in the field, and to put forth one viewpoint about the importance of the developmental changes in NMDAR subunit composition. Readers are directed to other recent reviews for a more in-depth perspective on the functions and regulation of NMDARs (Cull-Candy and Leszkiewicz, 2004; Kopp et al., 2007; Lau and Zukin, 2007) and for additional hypotheses regarding the induction of metaplasticity (Abraham, 2008).
2. Characteristics of NR2A and NR2B subunits
Among the six regulatory subunits of NMDARs, NR2A and NR2B have been the most extensively studied because they are broadly expressed in the brain, predominate in the postnatal cortex, and are believed to play important roles in synaptic plasticity. NR2A and NR2B subtypes of NMDARs are present as either di-heteromers (NR1/NR2A or NR1/NR2B) or tri-heteromers (NR1/NR2A/NR2B). A recent biochemical study involving serial immunoprecipitation from hippocampal lysates in young rats at P42 estimated that about two-thirds of NR2A and NR2B subunits are associated in NR1/NR2A or NR1/NR2B di-heteromeric complexes, and one third of NMDARs are NR1/NR2A/NR2B tri-heteromers (Al-Hallaq et al., 2007), although these proportions likely shift dramatically over development. There is great heterogeneity in the NMDAR subunit composition among dendritic spines, because the rate of blockade of NMDAR currents by the NR2B-specific antagonist, ifenprodil, are different among dendritic spines (Sobczyk et al., 2005). NR2A and NR2B differ in channel kinetics, synaptic localization, and protein binding partners, all of which are expected to influence the induction of synaptic plasticity (Table 1), elaborated as follows:
Table 1.
Comparison of NR2A and NR2B-containing NMDARs
| NR2A | NR2B | Reference | |
|---|---|---|---|
|
| |||
| Open probability | High | Low | Chen et al., 1999 |
| Erreger et al., 2005 (but also see Prybylowski et al., 2002) | |||
|
| |||
| Deactivation | Fast | Slow | Erreger et al., 2005 |
|
| |||
| Peak current | High | Low | Erreger et al., 2005 |
|
| |||
| Rise time | Fast | Slow | Chen et al., 1999 |
| Monyer et al., 1994 | |||
|
| |||
| Decay time | Fast | Slow | Prybylowski et al., 2002 |
| Vicini et al., 1998 | |||
|
| |||
| Charge transfer | Low | High | Erreger et al., 2005 |
|
| |||
| Ca2+/EPSC | Low | High | Sobczyk et al., 2005 |
|
| |||
| Location | Central synapse | Peri-synapse | Dalby and Mody, 2003 |
| Townsend et al., 2003 | |||
| Zhao and Constantine-Paton, 2007 | |||
|
| |||
| CaMKII binding | Weak | Strong | Mayadevi et al., 2002 |
| Strack and Colbran, 1998 | |||
|
| |||
| Plasticity | LTD/ltp | ltd/LTP | Barria and Malinow, 2005 |
| Berberich et al., 2005 | |||
| Morishita et al., 2006 | |||
| Philpot et al., 2007 | |||
| Tang et al., 1999 | |||
| Weitlauf et al., 2005 | |||
| Zhao et al., 2005 (but see Massey et al. 2004, Liu et al. 2004) | |||
Channel kinetics
At a macroscopic level, NR1/NR2A di-heteromeric channels exhibit faster rising and decaying currents than NR1/NR2B di-heteromeric channels (Chen et al., 1999; Monyer et al., 1994; Prybylowski et al., 2002; Vicini et al., 1998). NR1/NR2A/NR2B tri-heteromeric channels appear to exhibit intermediate decay time courses between the two di-heteromeric channel types (Vicini et al., 1998). The difference in the receptor decay kinetics between NR2A and NR2B receptor subtypes arises from their single channel behaviors. That is, NR1/NR2A channels have higher open probability and faster deactivation than NR1/NR2B channels (Chen et al., 1999; Erreger et al., 2005). Therefore, in response to glutamate release, NR1/NR2A channels tend to open and close earlier than NR1/NR2B channels, resulting in the faster rise and decay times observed macroscopically for NR2A-containing NMDARs (Chen et al., 1999; Erreger et al., 2005). Although NR1/NR2B channels may have lower peak currents, they carry about two-fold more charge for a single synaptic event than NR1/NR2A channels (Erreger et al., 2005). This occurs because deactivation of NR1/NR2B receptors is slow enough to compensate for their lower open probability (Erreger et al., 2005). Moreover, Ca2+ imaging studies suggest that NR2B-containing NMDARs carry more Ca2+ per unit of current than NR2A-containing NMDARs (Sobczyk et al., 2005). Therefore, NR1/NR2B channels may carry a greater Ca2+ charge than NR1/NR2A receptors because of their higher charge transfer and Ca2+ permeability. However, we stress that this viewpoint remains highly speculative, as this interpretation awaits studies both that directly measure Ca2+ responses in isolated NR2A-only or NR2B-only synapses and experiments that more accurately assess and quantify the open probability statistics of NR1/NR2B and NR1/NR2A receptors in mammalian neurons (see Prybylowski et al., 2002). Moreover, it is possible that NR2A-containing NMDARs may carry more of a Ca2+ charge than NR2B-containing NMDARs during high rates of synaptic activity, due to the higher open probability of NR2A subtypes (Erreger et al., 2005), although this needs to be examined in an intact neuronal preparation.
Synaptic localization
NMDARs are found both at synaptic and extrasynaptic sites including the cell soma and dendritic shaft. Extrasynaptic NMDARs are activated not only at pathological situations (Hardingham et al., 2002), but also by bursts of activity that can occur under physiological situations (Harris and Pettit, 2008). Therefore, extrasynaptic NMDARs are involved in stimulus-dependent synaptic modifications, and they are likely activated in a manner that is distinct from the activation of synaptic NMDARs. Synaptic and extrasynaptic NMDARs are shown to couple to distinct intracellular signaling pathways (Ehlers, 2003; Hardingham et al., 2002; Ivanov et al., 2006). Perhaps as a result of activating different signaling pathways, there is some evidence that synaptic NMDARs support LTP, while extrasynaptic NMDARs mediate LTD in the mature brain (Lu et al., 2001b; Massey et al., 2004), although this remains controversial.
The possibility that the subunit composition of NMDARs differs between synaptic and extrasynaptic sites is also controversial. It has been proposed that NR2A-containing NMDARs are more likely to occupy the central portion of the synapse, while NR2B-containing NMDARs are preferentially targeted to peripheral portions of the synapse or to extrasynaptic sites. This view has arisen from the finding that, in rat dentate gyrus granule cells, NMDAR-mediated miniature excitatory postsynaptic currents (mEPSCs) reveal faster decay kinetics than evoked NMDAR-mediated EPSCs (Dalby and Mody, 2003). Moreover, spontaneous neurotransmitter release fails to activate NMDAR currents in the midbrain of NR2A knockout mice, while evoked synaptic activity can drive NMDAR currents in the absence of NR2A (Townsend et al., 2003; Zhao and Constantine-Paton, 2007). These results suggest that glutamate from spontaneous transmitter release reaches only the central part of synapses, where NR2A-containing receptors are concentrated, while glutamate release from action potential driven synaptic transmission can also reach more peripheral NMDARs, where NR2B-containing NMDARs may be restricted to after a certain stage of development.
This view, however, was challenged by recent experiments conducted using the glutamate-uncaging technique. In these experiments, sensitivity to NR2B specific NMDAR antagonists are shown to be comparable between synaptic and extrasynaptic sites (Harris and Pettit, 2007). Furthermore, studies using NMDA bath applications to study extrasynaptic NMDAR subunit compositions have given conflicting results. Whereas some studies suggest that NR2B-containing NMDARs are most prevalent at extrasynaptic sites (Scimemi et al., 2004; Stocca and Vicini, 1998; Tovar and Westbrook, 1999), other studies suggests that both NR2A- and NR2B-containing NMDARs exist extrasynaptically (Mohrmann et al., 2000) and that the ratio of the two subtypes is comparable to that of synaptic NMDARs (Thomas et al., 2006). Some of these apparent discrepancies may be resolved by examining developmental and regional differences in the localization of NMDAR subtypes (Kohr, 2007).
Although extrasynaptic NMDARs are exposed to ambient glutamate, whether this glutamate concentration is high enough to tonically activate extrasynaptic NMDARs remains controversial. If extrasynaptic NMDARs are active, they may serve as leak channels that amplify excitatory inputs (Sah et al., 1989) and regulate synaptic plasticity (Izumi et al., 2008). Although microdialysis studies report that ambient glutamate concentrations in vivo are high enough to activate extrasynaptic NMDARs (Baker et al., 2002; Lerma et al., 1986; Nyitrai et al., 2006), a recent study suggests that glutamate transporters regulate ambient glutamate concentrations at a level that is too low to cause significant receptor activation (Herman and Jahr, 2007). Further studies are clearly needed to clarify the extent to which extrasynaptic NMDARs are activated by ambient glutamate.
Protein interaction
To induce NMDAR-dependent LTD and LTP, downstream signaling pathways have to tightly couple to NMDARs (reviewed in Kennedy et al., 2005). Proteins interacting with NMDAR subunits are therefore important determinates for the direction of synaptic plasticity. NR2A and NR2B each interact with different intracellular proteins. NR2B has many unique or preferential binding partners. For example, NR2B interacts directly with Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) (Krapivinsky et al., 2003), although whether this interaction occurs at synapses needs to be shown. NR2B is also linked indirectly to synaptic Ras GTPase activating protein (RasGAP), presumably through synapse-associated protein 102 (SAP102) (Kim et al., 1998). The unique associations of NR2B to Ras-GRF1 and RasGAP are likely to affect the induction of plasticity (Zhu et al., 2002).
One of the most important NMDAR binding partners is CaMKII, which is present in high levels at synapses (Erondu and Kennedy, 1985; Peng et al., 2004) and has a well-documented role in the induction of LTP (reviewed in Lisman et al., 2002). CaMKII binds with high affinity to NR2B subunits (Leonard et al., 1999; Strack and Colbran, 1998; Strack et al., 2000), and, to a much lesser extent, to NR2A subunits (Gardoni et al., 1999; Strack and Colbran, 1998). Ca2+ entering through NMDARs associates with the Ca2+ binding protein, calmodulin, and the Ca2+/calmodulin complex interacts with and activates CaMKII. Activated CaMKII binds strongly to NR2B (Strack and Colbran, 1998), allowing CaMKII to remain active even after dissociating from Ca2+/calmodulin (Bayer et al., 2001). Importantly, CaMKII activation and its association to NR2B are required for LTP induction (Barria and Malinow, 2005). Thus, LTP induction might rely heavily upon CaMKII being tethered closely to sites of Ca2+ entry via NR2B.
NR2A also appears to have some unique associations with signaling molecules. A recent study suggests that NR2A co-immunoprecipitates with neuronal nitric oxide (NO) synthase more effectively than NR2B (Al-Hallaq et al., 2007). Although this interaction is likely indirect, the association raises the interesting possibility that NO-mediated presynaptic forms of LTP and LTD (Haghikia et al., 2007; Prast and Philippu, 2001; Zhang et al., 2006) may be preferentially linked to NR2A-mediated signaling pathways.
Both NR2A and NR2B possess PDZ-binding motifs in their c-terminus. Through the PDZ-binding motifs, they interact with membrane-associated guanylate kinase (MAGUK) family of synaptic scaffolding proteins that in turn associate with important synaptic signaling molecules and tether NMDARs to intracellular signaling pathways (Kennedy, 2000). The differential interaction of NR2A and NR2B subunits to MAGUKs is controversial. It was once believed that NR2A preferentially bound to postsynaptic density protein-95 (PSD-95), while NR2B preferentially bound SAP102 (Sans et al., 2000; Townsend et al., 2003). Moreover, these interactions were thought to control distinct synaptic localization of NR2A and NR2B (Townsend et al., 2003). However, a recent biochemical study using a serial immunoprecipitation suggests that MAGUK proteins such as PSD-95 and SAP102 interact with di-heteromeric NR1/NR2A and NR1/NR2B receptors at comparable levels (Al-Hallaq et al., 2007). Additional studies are needed to clarify the association of NMDAR subunits with MAGUK family members and what effects these associations may have on receptor localization and on plasticity signaling pathways.
3. Activity-dependent modulation of NR2A/NR2B ratio
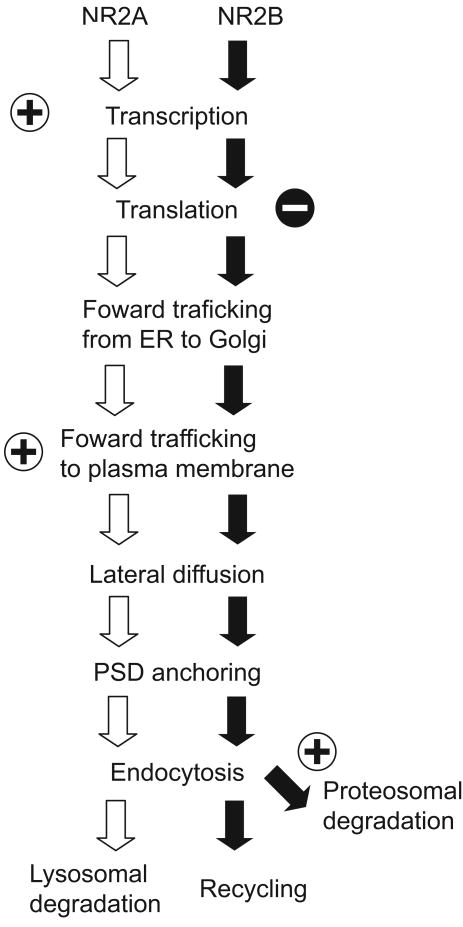
The NR2A/NR2B ratio is not fixed at synapses, rather, it changes with development (Flint et al., 1997; Sheng et al., 1994), sensory experience (Carmignoto and Vicini, 1992; Hestrin, 1992; Liu et al., 2004b; Nase et al., 1999; Quinlan et al., 1999a; Roberts and Ramoa, 1999), and synaptic plasticity (Bellone and Nicoll, 2007; Sobczyk and Svoboda, 2007). These changes may help to optimize the threshold for inducing synaptic plasticity at different developmental points and/or under different sensory environments (discussed later). An important question is how sensory experience and neuronal activity regulate the ratio of the two subunits. Recent studies in cultured neurons have revealed differential regulation of NR2A and NR2B subunits at various points in their synthesis, trafficking, and degradation. Here we describe mechanisms that control NR2A and NR2B at each step and how these mechanisms can be regulated by neuronal activity (Figure 1).
Figure 1. Activity-dependent regulation of NR2A and NR2B.
NR2A and NR2B contain distinct signals that control synaptic presentation of NMDARs. Whereas neuronal activity facilitates (+) transcription of NR2A subunit, it attenuates translation of NR2B (-). Neuronal activity also facilitates surface delivery of NR2A and proteasomal degradation of NR2B. (See text for details)
Transcription and translation
Much of what we understand of the transcriptional and translational regulation of NMDARs comes from studies of cultured neurons, and such studies reveal important differences based on the maturity of the neurons in culture. The early developmental increase (days in vitro (DIV) 9 to 15) in the NR2A/NR2B ratio is largely due to an increase in NR2A mRNA (Hoffmann et al., 2000), suggesting that the developmental shift is controlled at a transcriptional level (but also see (Follesa and Ticku, 1996). This increase in NR2A levels can be suppressed by a blocker of NMDARs ((2R)-amino-5-phosphonovaleric acid; APV), or an inhibitor of voltage-gated Ca2+ channels (Nifedipine). NR2B mRNA levels in immature cultures are insensitive to APV treatment. These data indicate that the early developmental increase in the NR2A/NR2B ratio is primarily due to an increase in NR2A levels driven by activity-dependent activation of NMDARs. These observations also suggest that NR2A and NR2B transcripts may be regulated by distinct Ca2+-dependent mechanisms. The basis for the differential transcriptional response for the NR2 subunits is currently unknown. However, analysis of transcriptional regulatory sequences of NR2A revealed that a sequence between nucleotides -1253 and -1180 in the upstream promoter region of NR2A contains a cAMP response element (CRE)-like element and is necessary for the developmental increase in NR2A (Desai et al., 2002). Although it has not yet been tested if this coding sequence is required for activity-dependent NR2A transcription, the possible regulation of NR2A by CRE gives rise to the interesting possibility that the activity-dependent developmental increases in NR2A could be mediated by the NMDAR/PKA/CREB pathway.
In older cortical cultures (DIV22-30), responses of NR2A and NR2B to APV treatment is quite different than that observed in younger cultures. At this stage, one day of NMDAR blockade by APV increases NR2B protein without affecting the level of NR2A protein (Chen and Bear, 2006). This NR2B increase is largely blocked by the translational inhibitors, cycloheximide and anisomycin (Chen and Bear, 2006). Therefore, NMDAR activity in more mature neurons may tonically suppress NR2B translation, and brief (one day) blockade of NMDARs may be sufficient to relieve this suppression. Thus, neuronal activity appears to facilitate transcription of NR2A in immature neurons, while activity and NMDAR activation suppresses translation of NR2B in more mature neurons. Importantly, both of these mechanisms increase the NR2A/NR2B ratio in response to enhanced neural activity.
Forward trafficking
NMDAR subunits are assembled in the endoplasmic reticulum (ER) (Qiu et al., 2005), modified in the ER and Golgi apparatus, and then trafficked to the plasma membrane. Both NR2A and NR2B contain an ER export signal (HLFY) at the base of their c-terminus (Hawkins et al., 2004). Because of the ER export signal on NR2 subunits, overexpression of NR2 subunits can overcome an ER retention signal located in NR1 subunits and enhance the surface delivery of NMDARs (Scott et al., 2001; Standley et al., 2000). In this regard, the increase in NR2B levels induced by NMDAR blockade (Chen and Bear, 2006) could also facilitate the surface delivery of NMDARs in mature neurons. Consistent with this idea, chronic inactivation of NMDARs has been shown to increase synaptic levels of NR1 (Crump et al., 2001; Rao and Craig, 1997) and NR2B (Ehlers, 2003).
Distinct activity-dependent mechanisms regulate the synaptic delivery of NR2A and NR2B (Barria and Malinow, 2002). Synaptic accumulation of NR2A-containing NMDARs requires glutamate binding to NMDARs, whereas NR2B-containing NMDARs can accumulate at synapses regardless of synaptic activity level or ligand binding (Barria and Malinow, 2002). These results provide further evidence that synaptic activity preferentially drives NR2A-containing NMDARs to the synapse. Thus, neuronal activity not only increases transcription of NR2A (Hoffmann et al., 2000) but also facilitates synaptic delivery of NR2A-containing NMDARs over NR2B-containing NMDARs. This differential regulation of NR2A and NR2B subunits ensures an activity-dependent increase in the NR2A/NR2B ratio.
Surface diffusion
Although it has been traditionally thought that NMDARs are relatively stable and immobile at the cell surface, recent electrophysiological and imaging studies suggest that NMDARs are highly mobile. By taking advantage of the irreversible open channel blocker, MK-801, Tovar and Westbrook have shown in immature cultured hippocampal neurons that synaptic NMDAR-mediated EPSCs can quickly recover following MK-801 block of synaptically evoked NMDARs (2002). This recovery was due to lateral diffusion, by which 65% of synaptic NMDARs exchange with extrasynaptic NMDARs in less than 7 minutes. NMDAR lateral mobility has also been observed by single molecule tracking (Groc et al., 2004; 2006; 2007). These studies reveal that NMDARs are highly mobile at both synaptic and extrasynaptic membranes. Importantly, the surface mobility of NMDARs appears to change with development (Harris and Pettit, 2007; Kohr, 2007) in a subunit composition-specific manner (Groc et al., 2006). NR2A-containing NMDARs are less mobile than NR2B-containing NMDARs, and the synaptic residency time of NR2B-containing NMDARs decreases over development. This decrease in synaptic dwell time of NR2B-containing NMDARs is mediated by Reelin, an extracellular matrix protein (Groc et al., 2007). Interestingly, the lateral mobility of NMDARs is insensitive to acute changes in neuronal activity levels, and this is unlike AMPARs whose diffusion is bidirectionally controlled by neuronal activity (Groc et al., 2004). It is unknown, however, if chronic activity manipulations for several days affects NMDAR surface mobility and thereby contributes in a homeostatic fashion to differential synaptic accumulations of NR2 subunits. It is important to note that these studies were performed primarily in neuronal cultures, where the packing density of cells is lower than that in slices or in vivo. Therefore, surface mobility of these receptors needs to be examined in more intact preparations.
Endocytosis
The ability of NMDARs to undergo endocytosis decreases with age (Roche et al., 2001). This developmental change is likely a consequence of the fact that NR2B subunits experience more robust endocytosis than NR2A subunits (Lavezzari et al., 2004), and the proportion of NR2B subunits decreases with age. How are the distinct endocytic mechanisms of NR2A and NR2B controlled? Both NR2A and NR2B contain a PDZ binding motif (ESDV) at the c-terminus (Lin et al., 2004; Prybylowski et al., 2005). This motif helps to tether the subunits to the postsynaptic density through binding to MAGUK proteins, such as PSD-95 and SAP102. Interestingly, PDZ binding is required for synaptic localization of NR2B, but not NR2A (Lin et al., 2004; Prybylowski et al., 2005). The c-termini of both NR2A and NR2B contain the endocytic signals LL (Lavezzari et al., 2004) and YEKL (Roche et al., 2001), respectively, which bind the AP2 clathrin adaptor protein to initiate clathrin-dependent endocytosis. Additional regulation of NR2B endocytosis is endowed through phosphorylation of tyrosine 1472 in YEKL by the Src-family kinase Fyn (Prybylowski et al., 2005). This phosphorylation protects YEKL from AP2 binding, and consequently limits NR2B subtypes from clathrin-dependent endocytosis. Thus, localization of NR2B-containing NMDARs to the postsynaptic density is controlled by Fyn and an unidentified phosphatase which removes the phosphate from YEKL. Such phosphorylation-dependent regulation of NR2A endocytosis has not yet been reported.
NR2 subunits can themselves dictate the fate of endocytosed NMDARs. Both NR2A and NR2B contain proximal motifs that direct NMDARs to the late enodosome/lysosome, where the receptors are degraded. However, NR2B subunits possess an additional proximal motif, which can help drive the receptors along a recycling pathway (Scott et al., 2004). As a consequence, endocytosed NR2B-containing NMDARs are preferentially recycled back to the plasma membrane surface, whereas NR2A di-heteromeric NMDARs are more likely to be degraded when endocytosed.
Whether activity-dependent endocytosis of NMDARs (Morishita et al., 2005) is regulated in a subunit-specific manner has not been examined. Given the recent finding of the rapid increase in the NR2A/NR2B ratio upon LTP induction in hippocampus (Bellone and Nicoll, 2007), it is tempting to speculate that LTP-inducing stimulation preferentially induces endocytosis of NR2B-containing NMDARs and/or insertion of NR2A-containing NMDARs.
Degradation
The ratio of NR2A/NR2B subunits can be bidirectionally regulated in response to alterations in neuronal activity levels, and this activity-dependent regulation can be prevented by proteasome inhibitors (Ehlers, 2003). This indicates that degradation pathways are important for the regulation of NMDAR subunit levels. A recent study showed that NR2B can be ubiquitinated by an E3 ligase, Mind bomb 2 (Mib2), thus tagging NR2B for proteasomal degradation (Jurd et al., 2007). Mib2 interacts directly with NR2B and ubiquitinates it when tyrosine 1472 in YEKL is phosphorylated by Fyn. Because neuronal activity facilitates tyrosine phosphorylation of NR2B and Mib2 binding (Jurd et al., 2007), Mib2 may play an important role in the activity-dependent regulation of NR2 subunits. In an apparent paradox, phosphorylation at tyrosine 1472 both increases the proteasomal degradation of NR2B but limits NR2B endocytosis (Prybylowski et al., 2005). Thus, it will be interesting to discern how synaptic localization and Mib2-mediated degradation of tyrosine 1472 phosphorylated-NR2B are balanced. Interestingly, although ubiquitination of NR2A has been observed (Monyer et al., 1994; Rezvani et al., 2007; Sheng et al., 1994), an E3 ligase that targets NR2A has not yet been reported.
In conclusion, NR2A and NR2B undergo differential activity-dependent regulation at various points of the protein synthesis, trafficking, and degradation pathways. It is thus clear that multiple layers of regulation contribute to the experience-dependent modifications of the synaptic NR2A/NR2B ratio.
4. Developmental and experience-dependent modification of the NR2A/NR2B ratio in vivo
Developmental regulation
In many parts of the CNS, including the brain stem, hippocampus, and neocortex, the NR2A/NR2B ratio increases during early postnatal development (Barth and Malenka, 2001; Chen et al., 2000; Hestrin, 1992; Liu et al., 2004b; Nase et al., 1999; Quinlan et al., 1999a; Roberts and Ramoa, 1999; Yoshimura et al., 2003). This change can occur both at the mRNA (Liu et al., 2004b; Nase et al., 1999) and protein levels (Chen et al., 2000; Quinlan et al., 1999a; Roberts and Ramoa, 1999). Furthermore, a profound increase in NR2A-containing NMDARs, rather than a decrease in NR2B subunits, is believed to be the primary factor contributing to the observation that the decay of NMDAR-mediated currents becomes faster with development (Carmignoto and Vicini, 1992; Quinlan et al., 1999a; Yoshimura et al., 2003). However, an increase in the NR2A/NR2B ratio may not be the sole factor that regulates NMDAR decay kinetics. For example, changes in NMDAR phosphorylation and the expression of NR1 splice variants also regulate NMDAR current kinetics (Lieberman and Mody, 1994; Rumbaugh et al., 2000; Tong et al., 1995). In some regions, the largest developmental decline in NMDAR-mediated current duration can actually precede the most profound increases in the NR2A/NR2B ratio (Barth and Malenka, 2001). Moreover, a mild but significant developmental decrease in NMDAR-mediated current decay time is still observed in NR2A knockout mice (Lu et al., 2001a). Nonetheless, studies in NR2A knockout mice demonstrate that the upregulation of NR2A underlies the largest developmental changes in NMDAR current duration (Fagiolini et al., 2003; Lu et al., 2001a; Mierau et al., 2004).
Experience-dependent regulation
In some parts of the neocortex, including the primary visual cortex, the elevation of the NR2A/NR2B ratio is dependent upon the level of neuronal activity (Liu et al., 2004b; Nase et al., 1999; Quinlan et al., 1999a; Roberts and Ramoa, 1999). Sensory deprivation, such as dark-rearing, reduces the developmental shift in the NR2A/NR2B ratio in the regions of the brain subserving the deprived sensory modality (Carmignoto and Vicini, 1992; Philpot et al., 2001; Quinlan et al., 1999a). In the visual cortex, this experience-dependent control of the NR2A/NR2B ratio is not restricted to young animals. We and others have shown that 10 days of visual deprivation in adult rodents can reduce the NR2A/NR2B ratio in the visual cortex (He et al., 2006; Yashiro et al., 2005). Our data suggest that the ratio change may be restricted to perisynaptic sites in adults, unlike the experience-dependent modifications in NR2A/NR2B that can occur at synapses in young rodents (Yashiro et al., 2005). This indicates that visual experience can modify NR2A/NR2B protein expression levels throughout development, but the ability to control NMDARs at the central portion of the synapse may be restricted to young animals. The more limited ability to modify synaptic NMDARs in the adult brain could be related to the age-dependent decrease in the lateral mobility of NMDARs (discussed above) and/or a consequence of activity regulating NR2B levels rather than NR2A levels in adults (He et al., 2006), although these possibilities have not yet been tested.
How is the NR2A/NR2B ratio controlled by visual experience? There are currently conflicting reports on how visual experience regulates NR2A and NR2B at the mRNA level. For example, one comprehensive microarray analysis of mouse visual cortex revealed that both NR2A and NR2B mRNA levels are elevated in rodents reared in complete darkness until P27 compared to those in age-matched light-reared animals (Tropea et al., 2006). In contrast, single cell RT-PCR analysis of mRNA isolated from neurons in layer 4 of rat visual cortex showed that dark-rearing until P20 significantly retards the developmental increase in NR2A mRNA (Nase et al., 1999). These differences may be a subtle consequence of the ages studied, the techniques employed, or the species studied.
There is general agreement that visual experience increases the NR2A/NR2B ratio at the protein level, although there are some subtle disagreements as to whether neural activity regulates NR2A levels, NR2B levels, or both. Dark-rearing of rats until 6 weeks of age does not change NR2B protein levels in synaptoneurosome fractions, although this manipulation significantly reduces NR2A protein levels starting at 3 weeks of age compared to age-matched normally-reared rats (Quinlan et al., 1999a). Moreover, light exposure rapidly raises NR2A protein levels within one hour (Quinlan et al., 1999b). Reciprocally, 5 weeks of dark-rearing reduces NR2A protein levels without affecting NR2B protein levels in cats (Chen et al., 2000). Moreover, an immunohistochemical analysis reports that the NR2A protein reductions occur in all layers of the visual cortex in dark-reared rats (Tongiorgi et al., 2003). Collectively, these results indicate that NR2A is the target of sensory experience-dependent regulation, but NR2B is not. Contrary to these results, a recent study suggests that visual experience may regulate both NR2A and NR2B levels with a temporally regulated manner (Chen and Bear, 2006). That is, dark-rearing from a few days after birth initially increases NR2B levels (at the 4th week postnatally), but it decreases NR2A level later (at the 6th week postnatally). Therefore, both NR2A and NR2B protein levels can be targets of sensory experience-dependent regulation, and the two proteins are inversely regulated by experience.
We suggest that the visual experience-dependent increase in the NR2A/NR2B ratio is controlled by several cellular processes. The developmental NR2A increase is likely driven by activity-dependent facilitation in transcription (Hoffmann et al., 2000). The NR2B level may be chronically attenuated by activity-dependent suppression of its translation (Chen and Bear, 2006). Moreover, neuronal activity may limit NR2B levels by facilitating its degradation via the Mib2-mediated ubiquitin proteasome system (Jurd et al., 2007). In the absence of visual experience, NR2A mRNA synthesis is slowed, the suppression of NR2B translation is relieved, and NR2B degradation is attenuated. As a result, while visual deprivation decreases NR2A levels, it increases NR2B levels. Consequently, visual deprivation causes a significant reduction in the NR2A/NR2B ratio. Importantly, visual deprivation does not dramatically affect synaptic accumulation of NR1 (Quinlan et al., 1999a), suggesting that changes in synaptic NMDAR subtype, rather than number, might underlie most of the deprivation-induced changes in synaptic plasticity (discussed below). Detailed biochemical studies are required to dissect further the visual experience-induced transcriptional, translational, and posttranslational controls of NR2 subunits.
5. Are activity-dependent changes in the NR2A/NR2B ratio input-specific or global?
We have described that the NR2A/NR2B ratio changes in response to neuronal activity both in vitro and in vivo. An important question is whether the NR2A/NR2B ratio is controlled at the level of individual synapses or in a global, cell-wide manner. In other words, are changes in the NR2A/NR2B ratio restricted only to stimulated synapses or are the changes induced in a cell-wide manner? If the latter is true, it would predict that stimulation of a subset of synapses alters the properties of synaptic plasticity throughout a neuron, and such changes obviously have different consequences on dynamic modifications of synapses across a neuronal network.
Most studies that have addressed the regulation of NR2A/NR2B have employed global manipulations of synaptic function, such as chronic treatment with APV or dark-rearing. Because these slow alterations of the NR2A/NR2B ratio are, at least in part, controlled by transcription and translation of NR2 subunits, these slow changes are likely to be achieved by global control of the NR2A/NR2B ratio on the neuronal surface. There is evidence, however, that the NR2A/NR2B ratio may also be controlled in a local (input-specific) manner. For example, the NR2A/NR2B ratio is different between intercortical and intracortical synapses in layer (L) 5 pyramidal neurons in the cortex (Kumar and Huguenard, 2003). Moreover, the synaptic distribution of NR2B subunits in the adult mouse hippocampus is asymmetric between the apical and basal dendrites of single neurons (Kawakami et al., 2003). Such observations indicate that the NR2A/NR2B ratio must have a level of regulation at individual synapses. In support of this view, a recent study in the immature CA1 region of the hippocampus demonstrated that LTP-inducing stimulation gives rise to a rapid and input-specific increase in the NR2A/NR2B ratio (Bellone and Nicoll, 2007). Such rapid changes are unlikely to be regulated at the transcriptional or translational levels. Moreover, it has been shown that degree of LTP, which can be partly controlled by the NR2A/NR2B ratio, varies among spines (Matsuzaki et al., 2004). Therefore, we hypothesize that the NR2A/NR2B ratio is regulated both at synaptic and cellular levels. Global regulation of the NR2A/NR2B levels might regulate the threshold of synaptic plasticity across the cell to direct the acquisition of stimulus-selective response properties (Philpot et al., 1999; 2007), while a rapid, input-specific regulation of NR2A/NR2B levels might limit runaway potentiation on individual synapses.
6. Roles of NR2A and NR2B in LTD and LTP
LTP
Given that NR2B-containing NMDARs reveal longer currents (Monyer et al., 1994), carry more Ca2+ per unit of current (Sobczyk et al., 2005), and interact preferentially with CaMKII compared to NR2A-containing NMDARs (Strack and Colbran, 1998), it is tempting to speculate that NR2B subtypes are more likely to favor the induction of LTP compared to NR2A subtypes. A number of lines of evidence support this hypothesis, some of which are highlighted here. (1) Ifenprodil, an NR2B-specific antagonist, completely blocks LTP induced by a pairing protocol in immature hippocampal slice cultures, suggesting a critical requirement of NR2B subtypes for the induction of LTP (Barria and Malinow, 2005). (2) Overexpression of NR2A, and a presumptive replacement of NR2B subtypes with NR2A subtypes, attenuates the induction of LTP by a pairing protocol in immature hippocampal slice cultures (Barria and Malinow, 2005). (3) In the anterior cingulate cortex of six- to eight-week-old mice, an NR2B-specific antagonist blocks LTP elicited by either a pairing induction protocol or theta-burst stimulation (Zhao et al., 2005). (4) In thalamocortical synapses in the barrel cortex of postnatal day (P) 3-5 mice, ifenprodil blocks LTP induced by a pairing protocol (Lu et al., 2001a). (5) Genetic lesion of NR2A fails to abolish hippocampal LTP in P28 mice, suggesting that NR1/NR2B di-heteromic receptors are sufficient to induce hippocampal LTP (Berberich et al., 2005; Weitlauf et al., 2005). (6) Transgenic overexpression of NR2B enhances hippocampal LTP in 4–6-months old mice (Tang et al., 1999). (7) Transient overexpression of NR2B c-terminus, which blocks the NR2B and CaMKII interaction, attenuates hippocampal LTP in 3-4 months old mice (Zhou et al., 2007). Thus, in various regions of the brain, NR2B-containing NMDARs help to promote the induction of LTP induced by a variety of stimulation protocols.
Contrary to these findings, it has been reported that NR2A-containing, but not NR2B-containing, NMDARs mediate LTP (Liu et al., 2004a). Support for this hypothesis comes from experiments utilizing a new pharmacological tool, NVP-AAM077, which was believed to specifically block NR2A-containing NMDARs. In these experiments, NVP-AAM077 was shown to block hippocampal LTP in 3-4 weeks old rats (Liu et al., 2004a). Similar observations were made using this antagonist in the adult perihinal cortex (Massey et al., 2004). However, the specificity of NVP-AAM077 has been questioned. While NVP-AAM077 was reported to be 100 times more selective for NR1/NR2A channels than NR1/NR2B channels using a cell line exogenously expressing human NMDARs (Liu et al., 2004a), this antagonist is only 6-12 fold more effective for NR1/NR2A channels than NR1/NR2B channels in heterologously expressed rodent NMDARs (Berberich et al., 2005; Feng et al., 2004; Katagiri et al., 2007; Neyton and Paoletti, 2006; Weitlauf et al., 2005). The low selectivity of NVP-AAM077 in rodents is supported by the finding that NVP-AAM077 blocks more than 20% of the NMDAR current in hippocampal slices of NR2A-null mice at P28 even at a low (50 nM) concentration (Berberich et al., 2005). These findings suggest that specificity of NVP-AAM077 is not sufficient to determine the role of NR2A in synaptic plasticity.
While we suggest that the induction of LTP is more likely to be favored with NR2B subtypes than NR2A subtypes, we caution that the roles of these receptors must be carefully considered within a developmental and regional context. Moreover, NMDARs are not the sole determinants for inducing plasticity. For example, both signaling molecules and inhibitory inputs are important contributors to the induction of plasticity (Choi et al., 2002; Steele and Mauk, 1999), and these factors are well known to vary with region and developmental stage (Chattopadhyaya et al., 2004; Jiang et al., 2005; Morales et al., 2002; Yasuda et al., 2003).
LTD
Which subunits mediate LTD? In contrast to one study demonstrating that ifenprodil completely blocks hippocampal LTD (Liu et al., 2004a), studies in three independent laboratories consistently found that hippocampal LTD is insensitive to NR2B blockade by ifenprodil (Morishita et al., 2006). Another study even suggests that ifenprodil enhances the induction of LTD in the CA1 region of the hippocampus (Hendricson et al., 2002). These studies demonstrate that the induction of LTD does not require activation of NR2B-containing NMDARs. A caveat in these pharmacological studies is in the complex nature of ifenprodil (Neyton and Paoletti, 2006). While ifenprodil blocks NMDARs at high concentrations of glutamate, it may actually potentiate NMDAR currents at low glutamate concentrations (Kew et al., 1996). Thus, ifenprodil may affect synapses differently depending on the glutamate concentration in the synaptic cleft. Such complexities might partially underlie observations that ifenprodil alters synaptic weakening in some conditions but not others. For example, in the adult perirhinal cortex, the ifenprodil-dependence of LTD relies on the state of the synapse (Massey et al., 2004); ifenprodil blocks LTD that has been induced at a basal state, but the antagonist fails to block depotentiation (a form of LTD induced at recently potentiated synapses).
Studies in mice lacking NR2A have attempted to illuminate a possible role for NR2A in LTD. In the visual cortex, the standard 1 Hz stimulation protocol (900 pulses) induces LTD in wildtype mice but gives rise to LTP in NR2A knockout mice. On the other hand, 0.5 Hz stimulation (900 pulses) induces LTD in NR2A knockout mice comparable to wildtype mice (Philpot et al., 2007). Therefore, one hypothesis is that the threshold for inducing LTP is lowered and the window for inducing LTD is diminished in NR2A knockout mice, as activation of NR2B-containing di-heteromeric NMDARs allows greater Ca2+ entry than is possible through NR2A-containing NMDARs. Such a threshold change by deleting NR2A may not be universal in the brain, because 1 Hz stimulation induces LTD in NR2A knockout mice in the midbrain (Zhao and Constantine-Paton, 2007). Thus, the consequences of deleting NR2A may vary depending on age or brain region. Future studies that take advantage of either conditional NR2A deletion and/or more specific NR2A antagonists are needed to clarify the possible role of NR2A in LTD.
Taken together, it is tempting to speculate that synapses which possess a high NR2A/NR2B ratio favor the induction of LTD versus LTP by limiting Ca2+ entry through NMDARs. A caveat mentioned previously is that there is a need to definitively establish differences between NR2A and NR2B subtypes in open probability, as the Ca2+ signaling through these two receptor subtypes hinges critically on how they behave endogenously in mammalian neurons. Moreover, possible differences between NR2A and NR2B subunits with LTD-inducing signaling components, such as the PP1/PP2B (calcineurin) pathway, have not yet been examined.
Although PP1 is shown to be recruited to synapses upon synaptic stimulation (Morishita et al., 2001), a direct interaction of PP1 to NR2A, NR2B, or other postsynaptic density proteins has yet to be shown (to the best of our knowledge). These caveats not withstanding, the existing data suggest that a high NR2A/NR2B ratio favors the activity-dependent induction of LTD (but does not prevent the ability to induce LTP).
NR2A/NR2B ratio controls the LTD/LTP threshold
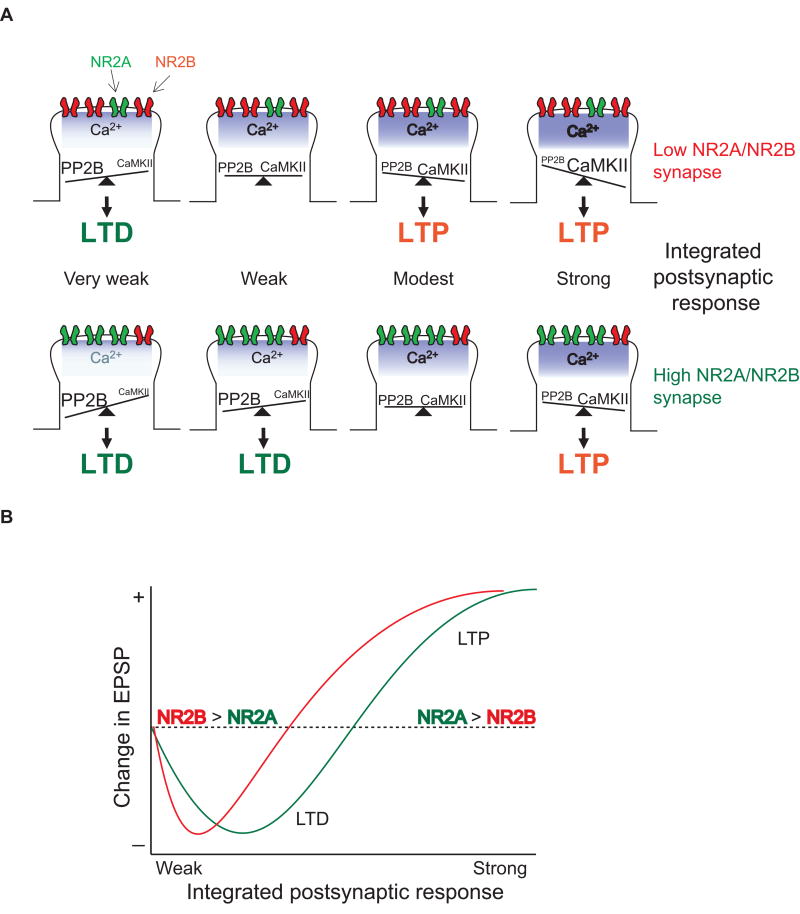
How do differences in the NR2A/NR2B ratio affect LTD and LTP? Given the contributions of NR2 subunits on LTD and LTP mentioned above, it has been hypothesized that the LTD/LTP crossover threshold is determined by the ratio of NR2A/NR2B expressed on dendritic spine surfaces (Figure 2)(Philpot et al., 1999). That is, if the ratio of NR2A/NR2B is elevated, stronger stimulation (e.g. a higher stimulus frequency) would be required to induce LTP compared to when the ratio of NR2A/NR2B is low, while a wider range of weaker stimulation (e.g. a broader range of low frequency stimulations) might be able to induce LTD. This hypothesis is based on two observations; a higher NR2A/NR2B ratio limits both the accessibility of CaMKII at the synapse and Ca2+ entry through NMDARs (although see Erreger et al., 2005). Therefore, a high NR2A/NR2B ratio would require a stronger postsynaptic response to elevate Ca2+ and activate CaMKII to a level sufficient to induce LTP. Likewise, weaker postsynaptic responses might suit activation of calcineurin and activate an LTD pathway (Philpot et al., 1999)(Figure 2B). Conversely, a low NR2A/NR2B ratio would lower the LTP induction threshold, making it more likely that a modest response can elevate Ca2+ and activate CaMKII to a level sufficient to induce LTP. Moreover, a low NR2A/NR2B ratio would reduce the LTD/LTP crossover threshold by elevating Ca2+ entry through NMDARs in response to stimulus trains. Consistent with the hypothesis that a low NR2A/NR2B ratio favors the induction of LTP, NR2B-containing receptors are preferentially expressed at smaller dendritic spines (Sobczyk et al., 2005), which are more likely to undergo LTP than larger spines (Matsuzaki et al., 2004). More directly, an experience-dependent upregulation in the NR2A/NR2B ratio increases the LTD/LTP crossover threshold in the visual cortex (discussed below).
Figure 2. Hypothetical model of synaptic plasticity regulation by NMDAR subunits.
A) A model to explain why the LTD/LTP induction threshold may differ between synapses with low (upper) and high (lower) NR2A/NR2B ratios. In these two synapses, the same frequencies of stimulation will produce different outcomes in synaptic plasticity, because of the difference in the relative level of activated PP2B and CaMKII, which stimulate LTD and LTP pathways, respectively. In synapses with a low NR2A/NR2B ratio (upper panels), large amounts of Ca2+ can enter the spine through NMDARs in response to synaptic stimulation and/or the calcium is more likely to activate CaMKII that is brought to the site of calcium entry via an interaction with the NR2B subunit. Therefore, modest synaptic activity is more likely to activate CaMKII and stimulate LTP pathways. With a low NR2A/NR2B ratio (upper panels), only very weak stimulation would activate calcineurin (PP2B) without sufficiently activating CaMKII, allowing LTD to be induced. Conversely, when NR2A-containing NMDARs dominate the postsynaptic membrane (lower panels), Ca2+ entry through NMDARs is limited and/or there is less CaMKII brought to the site of calcium entry via NR2B. This increases the stimulation requirements needed to activate CaMKII more than PP2B. Note that, in this model, both NR2A and NR2B-containing NMDARs are activated during the stimulation to induce LTD or LTP. The ratio of the two subunits receptors controls Ca2+ entry to spines or CaMKII sequestration, and hence the plasticity thresholds for LTD and LTP. Not depicted in this schematic is the fact that there is a level of postsynaptic activation below which synaptic plasticity is not induced, due to insufficient calcium entry.
B) Shematic depicting how NMDAR subunit regulates the properties of synaptic modification. The x-axis represents the level of the integrated postsynaptic response (which is related to the frequency of synaptic activation), while the y-axis represents the lasting change in synaptic strength. The curves are schematized from the data of (Kirkwood et al., 1996; Philpot et al., 2007; Philpot et al., 2003). When the synaptic NR2A/NR2B ratio is high, the LTD-LTP crossover point (θm) shifts to the right, decreasing the likelihood that LTP will occur. Conversely, when the synaptic NR2A/B ratio is low, θm slides to the left, favoring LTP over LTD.
While our model (Figure 2) depicts a static LTD threshold, theoretical analysis indicates that both the LTD and LTP thresholds will be shifted by a change in NMDAR efficacy (Castellani et al., 2001; Shouval et al., 2002). Probing the LTD induction threshold for frequency-dependent plasticity poses technical problems, due to the long baseline and induction protocols that are needed to test for the LTD threshold (discussed in Philpot et al., 2003). However, there are many examples in which both the LTD and LTP thresholds can be modified in a metaplasticity manner (reviewed in Abraham, 2008). For example, studies pairing voltage depolarizations with synaptic stimulation to induce LTD have shown that synapses have the capacity to modify the LTD threshold in an activity-dependent manner (e.g. Ngezahayo et al., 2000). Although changes in the LTD threshold have not been probed systematically with changes in the NR2A/NR2B ratio, it is likely that an increase in the NR2A/NR2B ratio would raise the LTD induction threshold and a decrease in the NR2A/NR2B ratio would lower the LTD induction threshold.
7. Interaction between the NR2A/NR2B ratio and LTD/LTP in the visual cortex
As discussed above, the NR2A/NR2B ratio increases during development and this increase can be regulated by sensory experience in many regions, including the visual cortex. Similar to the NR2A/NR2B ratio, properties of LTD and LTP change during development in an experience-dependent manner (Bear, 2003). Here we discuss if the described changes in LTD/LTP are mediated, in part, by the modification of the NR2A/NR2B ratio. We focus our discussion on two well-studied and mutually distinct excitatory synaptic connections within the visual cortex, the thalamus to layer 4 (thalamocortical) and the layer 4 to 2/3 (intracortical) connections. Interestingly, although the NR2A/NR2B ratio increases to a similar degree at both of these excitatory connections, the synapses reveal distinctive developmental and experience-dependent regulations of LTD and LTP.
Thalamocortical synapses
In the thalamocortical synapses of somatosensory and visual cortices, the magnitude of both LTD and LTP diminish in the second to fourth postnatal week in rodents (Dudek and Friedlander, 1996; Feldman et al., 1999; Jiang et al., 2007). Since the timing of the developmental increase in the NR2A/NR2B ratio coincides roughly with the loss of LTD and LTP in both the somatosensory (Barth and Malenka, 2001; Lu et al., 2001) and the visual cortex (Carmignoto and Vicini, 1992; Jiang et al., 2007; Quinlan et al., 1999b), it has been hypothesized that an increased NR2A/NR2B ratio may limit the expression of plasticity at thalamocortical synapses. This hypothesis has been challenged by a finding that the developmental reduction in thalamocortical LTP is conserved in the somatosensory cortex of NR2A knockout mice (Lu et al., 2001a). This suggests that the developmental increase in the NR2A/NR2B ratio does not mediate the developmental loss of thalamocortical LTP in the somatosensory cortex. Similarly, in the visual cortex, the current evidence indicates that the developmental loss of thalamocortical LTP in the visual cortex is also independent of the NR2A/NR2B switch. For example, while dark-rearing delays both the onset of the critical period for ocular dominance plasticity (Mower, 1991b), and the developmental upregulation of the NR2A/NR2B ratio at layer 4 (Carmignoto and Vicini, 1992; Quinlan et al., 1999a), this manipulation does not delay the developmental loss of thalamocortical LTP in the visual cortex (Jiang et al., 2007).
These data suggest that the NR2A/NR2B ratio is not the sole determinant for the ability to induce LTD and LTP at thalamocortical synapses. However, it is still conceivable that the relative levels of NR2A/NR2B might adjust the threshold for inducing plasticity within a developmental time point. For example, it is possible that an increase in the NR2A/NR2B ratio may increase the threshold for inducing LTP at thalamocortical synapses, but whether or not LTP can be induced at all is likely a consequence of other factors including inhibition (Kirkwood and Bear, 1994; Steele and Mauk, 1999), growth factors (Hu et al., 2007; Patterson et al., 1996), neuromodulators (Seol et al., 2007), or downstream plasticity signaling molecules (Yasuda et al., 2003). Future studies that manipulate NR2A and NR2B levels in vivo are needed to test these possibilities.
Intracortical synapses
Although a developmental loss of thalamocortical plasticity is generally accepted, it is less clear whether plasticity persists at the L4-L2/3 synapse, the first intracortical relay. There are reports that both LTD (Kirkwood et al., 1997) and LTP (Yoshimura et al., 2003) diminish at the L4-L2/3 synapses with development, but other studies find that LTD (Dudek and Friedlander, 1996; Jiang et al., 2007) and LTP (Frankland et al., 2001; Jiang et al., 2007; Kirkwood et al., 1997) persist into adulthood at this synapse. These seemingly contradictory findings make it difficult to determine whether observed developmental changes in the NR2A/NR2B ratio (Yoshimura et al., 2003) are associated with the ability to induce LTD and LTP at this intracortical synapse. The possibility that developmental changes in LTD and LTP are linked to changes in NMDAR subunit composition is further complicated by the fact that there are many developmental changes in molecules capable of altering synaptic plasticity (Sanes and Lichtman, 1999). For example, the Ca2+ buffering capability of neurons, which may change with development (Raza et al., 2007), alters the induction of synaptic plasticity. Thus, a host of developmental factors may be able to override or modify the impact of NR2A/NR2B levels. Existence of these factors are evident in adult hippocampus, where ifenprodil seems to have a relatively small effects on NMDAR-mediated EPSCs (Harris and Pettit, 2007; Lozovaya et al., 2004), suggesting few NR1/NR2B di-heteromeric receptors exist at this age, yet LTP could still be readily induced.
While the NR2A/NR2B ratio does not gate the absolute ability to induce LTD and/or LTP, changes in NR2A/NR2B appear to affect the threshold for the frequency-dependent induction of LTD/LTP. Visual experience/deprivation alters the frequency-response relationship of LTD/LTP in the layer 4-2/3 synapses, such that dark-rearing lowers the threshold for inducing both LTD and LTP, effectively increasing the window of stimulus frequencies that induce LTP (Kirkwood et al., 1996; Philpot et al., 2003). The observations that previous sensory experience modifies the properties of synaptic plasticity in the visual cortex is a well-documented effect that has been termed “metaplasticity” (Abraham and Bear, 1996; Philpot et al., 1999). Because dark-rearing reduces the NR2A/NR2B ratio, there is a striking correlation between the threshold for modifying synaptic strength and the relative ratio of NR2A/NR2B. Thus, it has been hypothesized that sensory experience slides the threshold for inducing LTD/LTP through regulation of the NR2A/NR2B ratio (Philpot et al., 1999), with a low NR2A/NR2B ratio favoring the induction of LTP (by lowering the LTD/LTP crossover threshold) (Fig. 2B).
We have taken advantage of NR2A knockout mice to test the hypothesis that the NR2A/NR2B ratio regulates the threshold of inducing synaptic plasticity. The idea of this study was to lock NMDAR subunit composition (by eliminating NR2A), as this should prevent experience-dependent modifications in the LTD/LTP threshold if this were normally a consequence of changing the NR2A/NR2B ratio. We first demonstrated that the visual experience-dependent shortening of NMDAR-current decay is absent in NR2A knockout mice (Philpot et al., 2007), indicating that the shortening of NMDAR currents is indeed due to an increase in the NR2A/NR2B ratio. Importantly, dark-rearing, which normally lowers the threshold for inducing LTP in wildtype mice, failed to alter the threshold for frequency-dependent plasticity in mice lacking NR2A. Moreover, the threshold stimulus frequency for inducing LTP is greatly lowered in NR2A knockout mice, such that 1Hz stimulation, which induces LTD in wildtype mice, is sufficient to induce LTP. These observations are consistent with the idea that a low NR2A/NR2B ratio favors the induction of LTP. Such a role for NMDAR subtypes in regulating plasticity induction has been observed in other regions of the brain, as either olfactory learning (Quinlan et al., 2004; Zinebi et al., 2003) or sleep deprivation (Kopp et al., 2006) can increase the ratio of NR2A/NR2B coincident with an increase in the induction threshold for LTP. These observations suggest that the alterations in NR2A/NR2B ratio might be a general neural mechanism for regulating the properties of synaptic plasticity.
The precise mechanism by which the NR2A/NR2B ratio alters the plasticity threshold is currently unknown. One possibility is that the NR2A/NR2B ratio is a critical regulator of Ca2+ entry through NMDARs. Alternatively, the NR2A/NR2B ratio might regulate plasticity by changing the relative complement of plasticity molecules brought to the synapse. For example, the unique association of NR2B with CaMKII could be a crucial mediator of the plasticity threshold (Barria and Malinow, 2005). Although the precise mechanism is poorly understood, it is clear that NR2A is required for metaplasticity in the developing visual cortex, and the visual experience-dependent changes in the NR2A/NR2B ratio mediate metaplasticity via the unique biophysical properties of each subunit.
It is currently unknown whether metaplastic changes associated with the developmental or experience-dependent increases in the NR2A/NR2B ratio are a consequence of a relative increase in NR2A di-heteromeric NMDARs, NR2A/NR2B tri-heteromeric NMDARs, or both. As we described previously, it has been estimated that 1/3 of synaptic NMDARs are tri-heteromeric receptors that contain both NR2A and NR2B (Al-Hallaq et al., 2007). It has also been shown electrophysiologically that NR1, NR2A, and NR2B can form tri-heteromeric receptors in cultured cells expression those three subunits. These tri-heteromeric receptors may contain properties bestowed upon them by both NR2A and NR2B, such as a high affinity for zinc and ifenprodil, respectively (Hatton and Paolletti, 2005). The macroscopic kinetics of these tri-heteromeric receptors have intermediate decay kinetics, although their kinetics more closely resemble those of the faster NR1/NR2A di-heteromers (Tovar and Westbrook, 1999; Vicini et al., 1998), and they lack strong block by ifenprodil (Hatton and Paolletti, 2005). Because it is currently impossible either to isolate tri-heteromeric currents or to block them selectively, it is essentially unknown how the tri-heteromeric receptors affect synaptic plasticity. We speculate that these receptors show intermediate Ca2+ permeability to the NR1/NR2A and NR1/NR2B di-heteromers, and these tri-heteromeric NMDARs may modestly associate with CaMKII through the binding to NR2B (Barria and Malinow, 2005). As such, we would further speculate that NR1/NR2A/NR2B receptors adjust the plasticity threshold to an intermediate level between the two extremes of having either a pure population of NR2B-only receptors (low LTP threshold) or a population of pure NR2A-only receptors (high LTP threshold). Because it is difficult to predict the impact that tri-heteromeric NMDARs would have on metaplasticity, it will be important both to systematically assess tri-heteromeric NMDAR content at the synapse and to develop novel approaches to selectively perturb or activate this unique population of receptors.
It is well known that prolonged activity-blockade in cultured neurons results not only in the decrease in the NR2A/NR2B ratio, but increase in NMDAR numbers at synapses (Mu et al., 2003; Rao and Craig, 1997; Watt et al., 2000). Therefore, one might predict that visual deprivation would increase total NMDAR number and reduce LTP induction threshold. This idea is attractive, but to date it has been observed that visual deprivation alters neither synaptic NR1 proteins (Quinlan et al., 1999a; Quinlan et al., 1999b) nor NMDAR-mediated mEPSC amplitudes (Carmignoto and Vicini, 1992), which reflect total receptor number at synapses. Therefore, changes in the total NMDAR content at the synapse may not be the endogenous mechanism for regulating experience-driven metaplasticity in the visual cortex, although this idea is in need of further testing.
8. Roles of NR2A and NR2B in cortical functions in vivo
We have described the roles of NR2A and NR2B in synaptic plasticity in vitro, but how do changes in these NMDAR types contribute to the development of sensory systems? Here we illustrate the roles of the NR2 subunits in the development of two well-studied visual functions: orientation selectivity and ocular dominance plasticity.
Orientation selectivity
Most neurons in the primary visual cortex respond vigorously to light-dark bars or edges presented to animals at a particular range of orientations. Some degree of orientation selectivity is innate in cortical neurons and the selectivity becomes more fine-tuned with development. Visual experience is necessary for the proper development of orientation selectivity, and fewer cells exhibit orientation selectivity in the absence of prior visual experience (Fagiolini et al., 2003; White et al., 2001). Moreover, if the visual environment is largely restricted to one orientation (e.g. by rearing in a striped cylinder) or if one orientation is presented repeatedly, animals develop orientation selectivity biased toward the orientation of the stripes (Frenkel et al., 2006; Sengpiel et al., 1999). These results indicate that cortical neurons change their connectivity to respond more to experienced orientations.
By what process do neurons progressively gain selective responses to external stimuli (such as the precise orientation of a bar of light)? One intuitive hypothesis is that patterned visual stimulation potentiates synapses of neurons, which gain preferential responses to repeatedly experienced orientations through an LTP-like mechanism. Conversely, the same stimulation may depress synapses responding to the non-favored orientation, via an LTD-like mechanism. The acquisition of stimulus selective properties such as orientation selectivity are thought to require experience-dependent modifications in the properties of synaptic plasticity (metaplasticity) (Abraham, 2008; Bienenstock et al., 1982). Thus, orientation selectivity is less likely to arise, and more likely to be broad when it does occur, in the absence of metaplasticity. Given that the NR2A/NR2B ratio controls visual cortex metaplasticity, one would predict that orientation selectivity would be severely retarded if the NR2A/NR2B ratio were fixed (hence preventing metaplasticity). Consistent with this hypothesis, the proportion of orientation selective neurons is severely diminished in the visual cortex of NR2A knockout mice (Fagiolini et al., 2003). It is tempting to speculate that, under normal conditions, a visual experience-dependent increase in the NR2A/NR2B ratio mediates the establishment of orientation selectivity by progressively widening the window for LTD induction and reducing the window for LTP induction (Fig. 2B). Thus, an LTP-like mechanism would maintain only highly orientation-specific synaptic connections.
Ocular dominance plasticity
One well-studied in vivo paradigm of synaptic plasticity is the ocular dominance shift observed in the primary visual cortex. Classical studies demonstrate that closure of one eye results in a loss of responsiveness to the closed (deprived) eye and increased responsiveness to the open (non-deprived) eye (Wiesel and Hubel, 1963). Recent studies, which investigate visually-evoked potential recordings in awake mice, reveal that such monocular deprivation (MD) starting at P28 induces depression of the closed eye cortical response in the first few days after MD, followed by a subsequent potentiation of the open eye response (Frenkel and Bear, 2004). Similar changes were observed using in vivo two-photon calcium imaging (Mrsic-Flogel et al., 2007). Are the initial depression and subsequent potentiation induced by LTD and LTP-like mechanisms, respectively? It has been shown that monocular deprivation for 24 hours in rats at P21-25 induces dephosphorylation of AMPARs, which is a molecular hallmark for LTD (Heynen et al., 2003). Moreover, LTD is suppressed in visual cortical slices prepared from monocularly deprived rats, suggesting that naturally occurring synaptic depression from monocular deprivation occluded the subsequent induction of LTD (Heynen et al., 2003). Thus, the closed eye depression seems likely to involve a LTD-like mechanism. Because the ocular dominance shift requires the autophosphorylation of CaMKII at threonine 286 (tested by monocular deprivation for 4 days starting at P26-30) (Taha et al., 2002), its expression mechanism shares common molecular pathways with LTP. It has not been investigated, however, if open-eye potentiation is absent in the CaMKII mutant mice.
Ocular dominance plasticity is most dramatic during a brief period of postnatal life, termed the critical period. In mice, this period lasts roughly from 3 to 5 weeks of age (Gordon and Stryker, 1996). Interestingly, at the onset of the critical period, NR2A protein levels markedly increase (Chen et al., 2000; Quinlan et al., 1999a; Roberts and Ramoa, 1999) and NMDAR decay kinetics decrease steeply in mice (Carmignoto and Vicini, 1992). This indicates that an increase in the NR2A/NR2B ratio may help to enable plasticity expressed to initiate the critical period. Consistent with this view, dark-rearing that delays the developmental increase in the NR2A/NR2B ratio also delays the initiation of the critical period (Mower, 1991a). Moreover, genetic deletion of NR2A suppresses ocular dominance plasticity without changing the timing or duration of the critical period (Fagiolini et al., 2003). Together, these findings suggest that experience-dependent increases in the NR2A/NR2B ratio may be required to enable certain forms of critical period plasticity. This hypothesis is attractive, because it predicts that, in order to have full expression of critical period plasticity, the NR2A/NR2B ratio needs to reach a sufficient level to promote weakening (e.g. LTD) of deprived eye inputs.
It was originally hypothesized that the increase in the NR2A/NR2B ratio regulates the end of the critical period (Carmignoto and Vicini, 1992; Fox and Zahs, 1994). But, as mentioned above, studies in NR2A knockout mice revealed that the developmental increase in NR2A is not essential for the end of the critical period either in the somatosensory (Lu et al., 2001a) or visual (Fagiolini et al., 2003) cortices. Despite this evidence, it is still premature to conclude that changes in NR2 subunits do not contribute to the termination of the critical period. Indeed, a loss of NR2B immunoreactivity at layer 4 tightly coincides with the end of the critical period in both somatosensory (Liu et al., 2004) and visual (Erisir and Harris, 2003) cortices. Therefore, although the end of the critical period is not regulated by changes in NR2A, it may instead be controlled by the decrease in NR2B at layer 4 synapses (independent of changes in NR2A). Because NR2B knockout mice die shortly after birth (Kutsuwada et al., 1995), future studies taking advantage of conditional NR2B deletion are needed to test the hypothesis that a sharp reduction in NR2B at thalamocortical synapses may terminate critical period plasticity.
Interestingly, recent findings suggest that the change in the NR2A/NR2B ratio could also be involved in the dynamic regulation of the ocular dominance shift. Although monocular deprivation initially causes depression of deprived-eye inputs following monocular deprivation, the subsequent potentiation of the open eye inputs takes 5 days after monocular deprivation (Frenkel and Bear, 2004). A recent biochemical study suggests that this delay may be a result of a deprivation-induced reduction in the NR2A/NR2B ratio (Chen and Bear, 2006). This reduction in NR2A/NR2B may lower the LTP induction threshold and allow weak ipsilateral inputs to induce LTP. This hypothesis explains why the potentiation of the open-eye response is slow to emerge compared to the rapid depression of the deprived eye (Frenkel and Bear, 2004). The above observations are consistent with a global regulation of NR2A/NR2B levels, as the reduced response at one set of inputs following deprivation eventually leads to a change in the LTP threshold at a second set of synapses corresponding to the open eye.
The above data indicate that the experience-dependent increase in the NR2A/NR2B ratio regulates the threshold for inducing plasticity. As such, the NR2A/NR2B switch is important both for the acquisition of stimulus-selective properties such as orientation selectivity and for the full expression of ocular dominance plasticity. Additionally, a change in the NR2A/NR2B ratio might underlie the naturally-occurring metaplasticity observed in visual cortex following monocular deprivation, with an initial deprivation-induced depression and a delayed potentiation of the open eye response. Genetic manipulation of NR2 subunits combined with chronic in vivo measurements will clarify the roles of NR2 subunits in synaptic plasticity in vivo.
9. Conclusion
Here we proposed that the ratio of NR2A/NR2B in synaptic NMDARs controls the threshold for synaptic modifications by controlling Ca2+ entry and intracellular signaling cascades. In our proposed model, NR2A-dominated synapses are more likely to induce LTD than NR2B-dominated synapses, while NR2B-dominated synapses have a greater capacity to be potentiated. However, both NR2A-containing and NR2B-containing NMDARs are capable of supporting bidirectional synaptic plasticity. This idea is largely consistent with the available data. It is important to bear in mind that LTD and LTP are complicated cellular processes involving many signaling proteins and are expressed by different mechanisms among brain regions and developmental stages (Malenka and Bear, 2004). Thus, although regulation of the NR2A/NR2B ratio is clearly a major determinant of the properties of synaptic plasticity, it is certainly only one of several important factors that affect the developmental and experience-dependent properties of synaptic plasticity.
The activity-dependent control of the NR2A/NR2B ratio provides another layer for regulating synapses in addition to activity-dependent modifications of synaptic strength. Since the NR2A/NR2B ratio affects induction thresholds for LTD and LTP, the synapse modification thresholds are controlled by sensory experience. Therefore, the NR2A/NR2B ratio changes occurring after synaptic stimulation or sensory experience alter how synapses change in response to subsequent synaptic stimulations or sensory experience. Thus, ongoing sensory experiences modify both the current state and the future destiny (predisposition) of synapses. This process endows neuronal networks not only with a feedback mechanism to adjust to an ever-changing environment, but also with a further competency in appropriately directing their rearrangement by sensory experience.
Acknowledgments
We thank Serena Dudek, Mike Ehlers, Lee Langer, Paul Manis, Sri Raghavachari, Adam Roberts, Robert Sealock, Ann Stuart, and Kazuhiro Wada for helpful discussions and critical readings of this review. Work that contributed to this review was supported by grants from the Whitehall Foundation and National Eye Institute (R01EY018323) to BDP and a University of North Carolina Dissertation Completion Fellowship to KY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham W, Bear M. Metaplasticity: The plasticity of synaptic plasticity. TINS. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaethetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Castellani GC, Quinlan EM, Cooper LN, Shouval HZ. A biophysical model of bidirectional synaptic plasticity: dependence on AMPA and NMDA receptors. Proc Natl Acad Sci U S A. 2001;98:12772–12777. doi: 10.1073/pnas.201404598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Cooper NG, Mower GD. Developmental changes in the expression of NMDA receptor subunits (NR1, NR2A, NR2B) in the cat visual cortex and the effects of dark rearing. Mol Brain Res. 2000;78:196–200. doi: 10.1016/s0169-328x(00)00076-0. [DOI] [PubMed] [Google Scholar]
- Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003;90:786–797. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- Desai A, Turetsky D, Vasudevan K, Buonanno A. Analysis of transcriptional regulatory sequences of the N-methyl-D-aspartate receptor 2A subunit gene in cultured cortical neurons and transgenic mice. J Biol Chem. 2002;277:46374–46384. doi: 10.1074/jbc.M203032200. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003;23:5208–5218. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci U S A. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. NMDA receptor upregulation: molecular studies in cultured mouse cortical neurons after chronic antagonist exposure. J Neurosci. 1996;16:2172–2178. doi: 10.1523/JNEUROSCI.16-07-02172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, van Dalen JJ, Gispen WH, Cattabeni F, Di Luca M. AlphaCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation. FEBS Lett. 1999;456:394–398. doi: 10.1016/s0014-5793(99)00985-0. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Recruiting Extrasynaptic NMDA Receptors Augments Synaptic Signaling. J Neurophysiol. 2008;99:524–533. doi: 10.1152/jn.01169.2007. [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Miao CL, Lippmann MJ, Morrisett RA. Ifenprodil and ethanol enhance NMDA receptor-dependent long-term depression. J Pharmacol Exp Ther. 2002;301:938–944. doi: 10.1124/jpet.301.3.938. [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H, Gremme T, Hatt H, Gottmann K. Synaptic activity-dependent developmental regulation of NMDA receptor subunit expression in cultured neocortical neurons. J Neurochem. 2000;75:1590–1599. doi: 10.1046/j.1471-4159.2000.0751590.x. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Tokuda K, Zorumski CF. Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase. Hippocampus. 2008;18:258–265. doi: 10.1002/hipo.20383. [DOI] [PubMed] [Google Scholar]
- Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–9652. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Thornton C, Wang J, Luong K, Phamluong K, Kharazia V, Gibb SL, Ron D. Mind bomb-2 is an E3 ligase that ubiquitinates the nmda receptor NR2B subunit in a phosphorylation-dependent manner. J Biol Chem. 2007 doi: 10.1074/jbc.M705580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science. 2003;300:990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Ganong AH, Brown T. Hebbian synapses in the hippocampus. Proc Natl Acad Sci USA. 1986;83:5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- Kew JN, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol. 1996;497(Pt 3):761–772. doi: 10.1113/jphysiol.1996.sp021807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of αCaMKII mutant mice. Proc Natl Acad Sci. 1997;94:3380–3383. doi: 10.1073/pnas.94.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor mobility: cultures versus acute brain slices or neonatal versus mature synapses? J Physiol. 2007;584:367. doi: 10.1113/jphysiol.2007.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. J Neurosci. 2003;23:10074–10083. doi: 10.1523/JNEUROSCI.23-31-10074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001a;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001b;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block the induction of hippocampal long term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau SB, Meredith RM, Upton AL, Paulsen O. Dissociation of experience-dependent and -independent changes in excitatory synaptic transmission during development of barrel cortex. Proc Natl Acad Sci U S A. 2004;101:15518–15523. doi: 10.1073/pnas.0402916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann R, Hatt H, Gottmann K. Developmental regulation of subunit composition of extrasynaptic NMDA receptors in neocortical neurones. Neuroreport. 2000;11:1203–1208. doi: 10.1097/00001756-200004270-00012. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991a;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Dev Brain Res. 1991b;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- Nase G, Weishaupt J, Stern P, Singer W, Monyer H. Genetic and epigenetic regulation of NMDA receptor expression in the rat visual cortex. Eur J Neurosci. 1999;11:4320–4326. doi: 10.1046/j.1460-9568.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngezahayo A, Schachner M, Artola A. Synaptic activity modulates the induction of bidirectional synaptic changes in adult mouse hippocampus. J Neurosci. 2000;20:2451–2458. doi: 10.1523/JNEUROSCI.20-07-02451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyitrai G, Kekesi KA, Juhasz G. Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr Top Med Chem. 2006;6:935–940. doi: 10.2174/156802606777323674. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Bear MF, Abraham WC. Metaplasticity: the plasticity of synaptic plasticity. In: Katz PS, editor. Beyond Neurotransmission: Neuromodulation and its importance for information flow. Oxford University Press; U.K., Oxford: 1999. [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory Role of NR2A for Metaplasticity in Visual Cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Hua YL, Yang F, Chen YZ, Luo JH. Subunit assembly of N-methyl-d-aspartate receptors analyzed by fluorescence resonance energy transfer. J Biol Chem. 2005;280:24923–24930. doi: 10.1074/jbc.M413915200. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Raza M, Deshpande LS, Blair RE, Carter DS, Sombati S, DeLorenzo RJ. Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons. Neurosci Lett. 2007;418:77–81. doi: 10.1016/j.neulet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EB, Ramoa AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2:597–604. doi: 10.1038/10154. [DOI] [PubMed] [Google Scholar]
- Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J Neurosci. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F, Stawinski P, Bonhoeffer T. Influence of experience on orientation maps in cat visual cortex. Nat Neurosci. 1999;2:727–732. doi: 10.1038/11192. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:10831–10836. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A, Svoboda K. Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron. 2007;53:17–24. doi: 10.1016/j.neuron.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Steele PM, Mauk MD. Inhibitory control of LTP and LTD: stability of synapse strength. J Neurophysiol. 1999;81:1559–1566. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Ferrero F, Cattaneo A, Domenici L. Dark-rearing decreases NR2A N-methyl-D-aspartate receptor subunit in all visual cortical layers. Neuroscience. 2003;119:1013–1022. doi: 10.1016/s0306-4522(03)00196-9. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Townsend M, Yoshii A, Mishina M, Constantine-Paton M. Developmental loss of miniature N-methyl-D-aspartate receptor currents in NR2A knockout mice. Proc Natl Acad Sci U S A. 2003;100:1340–1345. doi: 10.1073/pnas.0335786100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Postsynaptic control of hippocampal long-term potentiation. J Physiol Paris. 1986;81:228–236. [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci. 2005;25:11684–11692. doi: 10.1523/JNEUROSCI.4362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Constantine-Paton M. NR2A / Mice Lack Long-Term Potentiation But Retain NMDA Receptor and L-Type Ca2+ Channel-Dependent Long-Term Depression in the Juvenile Superior Colliculus. J Neurosci. 2007;27:13649–13654. doi: 10.1523/JNEUROSCI.3153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zinebi F, Xie J, Liu J, Russell RT, Gallagher JP, McKernan MG, Shinnick-Gallagher P. NMDA currents and receptor protein are downregulated in the amygdala during maintenance of fear memory. J Neurosci. 2003;23:10283–10291. doi: 10.1523/JNEUROSCI.23-32-10283.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]