Abstract
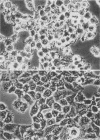
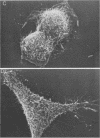
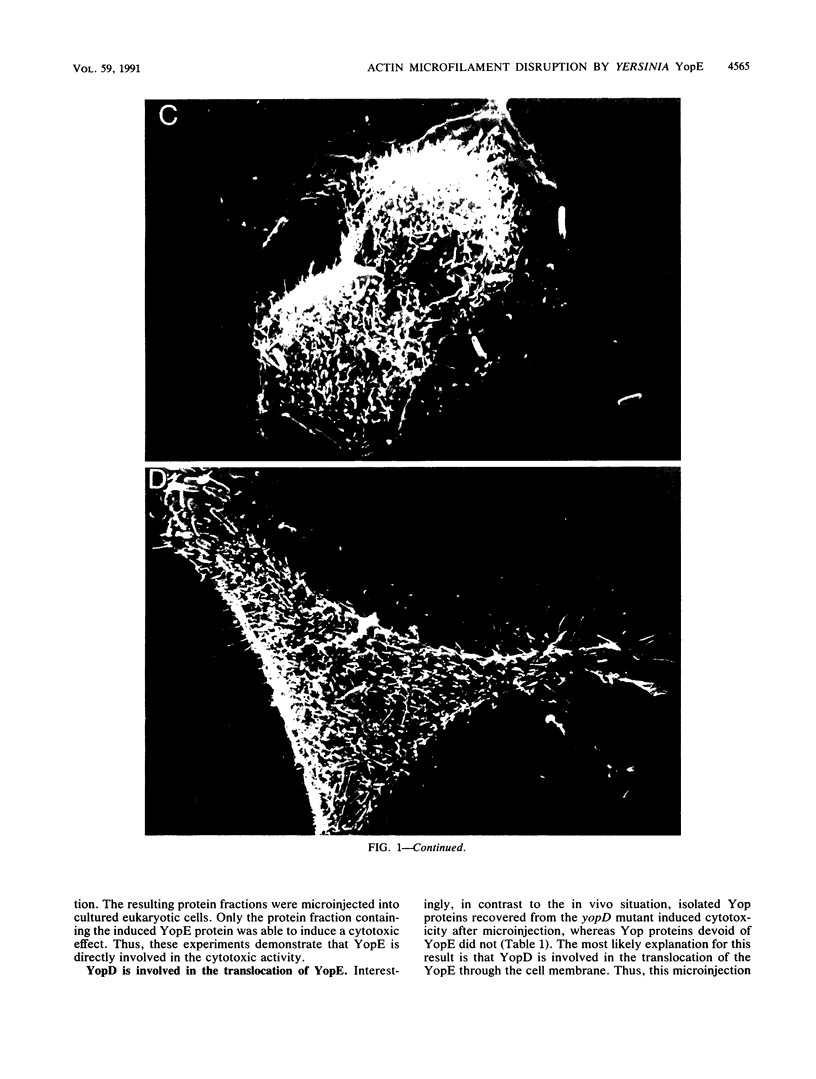
Pathogenic Yersinia spp., including the etiological agent of plague, Y. pestis, all carry a common plasmid that encodes a number of essential virulence determinants, the Yop proteins. One of these, YopE, has been shown to be involved in the obstruction of the primary host defense by a molecular mechanism leading to inhibition of phagocytosis (R. Rosqvist, A. Forsberg, M. Rimpiläinen, T. Bergman, and H. Wolf-Watz, Mol. Microbiol. 4:657-667, 1990). Although the Yop proteins are secreted into the culture supernatant in vast amounts, in vitro studies of the function of the Yop proteins have so far been unsuccessful. We show that isolated Yop proteins indeed can cause cytotoxic effects in vitro if the proteins are introduced intracellularly into the eukaryotic cell. Isolated Yop proteins of Yersinia pseudotuberculosis were found to disrupt the microfilament structure when microinjected intracellularly into the host cell. In particular, YopE was demonstrated to be directly involved in the cytotoxic action, whereas YopD seems to have a critical role in translocating the YopE protein through the host cell membrane. These results elucidate the requirement for at least some of the Yop proteins to leave the pathogen during infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Wegner A. ADP-ribosylation of actin by clostridial toxins. J Cell Biol. 1989 Oct;109(4 Pt 1):1385–1387. doi: 10.1083/jcb.109.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. Br J Exp Pathol. 1956 Jun;37(3):286–299. [PMC free article] [PubMed] [Google Scholar]
- Bergman T., Håkansson S., Forsberg A., Norlander L., Macellaro A., Bäckman A., Bölin I., Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991 Mar;173(5):1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Guan K. L., Dixon J. E., Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984 Jan;43(1):72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Bölin I., Norlander L., Wolf-Watz H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb Pathog. 1987 Feb;2(2):123–137. doi: 10.1016/0882-4010(87)90104-5. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. Genetic analysis of the yopE region of Yersinia spp.: identification of a novel conserved locus, yerA, regulating yopE expression. J Bacteriol. 1990 Mar;172(3):1547–1555. doi: 10.1128/jb.172.3.1547-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988 Jan;2(1):121–133. [PubMed] [Google Scholar]
- Goguen J. D., Walker W. S., Hatch T. P., Yother J. Plasmid-determined cytotoxicity in Yersinia pestis and Yersinia pseudotuberculosis. Infect Immun. 1986 Mar;51(3):788–794. doi: 10.1128/iai.51.3.788-794.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Hanski C., Naumann M., Grützkau A., Pluschke G., Friedrich B., Hahn H., Riecken E. O. Humoral and cellular defense against intestinal murine infection with Yersinia enterocolitica. Infect Immun. 1991 Mar;59(3):1106–1111. doi: 10.1128/iai.59.3.1106-1111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H., Lindberg U. The effect of divalent cations on the interaction between calf spleen profilin and different actins. Biochim Biophys Acta. 1988 Mar 2;953(1):95–105. doi: 10.1016/0167-4838(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Leung K. Y., Reisner B. S., Straley S. C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990 Oct;58(10):3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Pai C. H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985 Jul;49(1):145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Warder E. Glass beads load macromolecules into living cells. J Cell Sci. 1987 Dec;88(Pt 5):669–678. doi: 10.1242/jcs.88.5.669. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Kobayashi T., Shinoda S., Inoue T., Yukitake J., Shimizu K., Kawamoto Y., Moriyama T., Miyama A. Cytotoxicity and calcium-dependent antigen of Yersinia. Microbiol Immunol. 1984;28(1):33–49. doi: 10.1111/j.1348-0421.1984.tb02945.x. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg A., Rimpiläinen M., Bergman T., Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990 Apr;4(4):657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Wolf-Watz H. Virulence plasmid-associated HeLa cell induced cytotoxicity of Yersinia pseudotuberculosis. Microb Pathog. 1986 Jun;1(3):229–240. doi: 10.1016/0882-4010(86)90047-1. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Portnoy D. A., Bölin I., Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985 Apr;48(1):241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]