Abstract
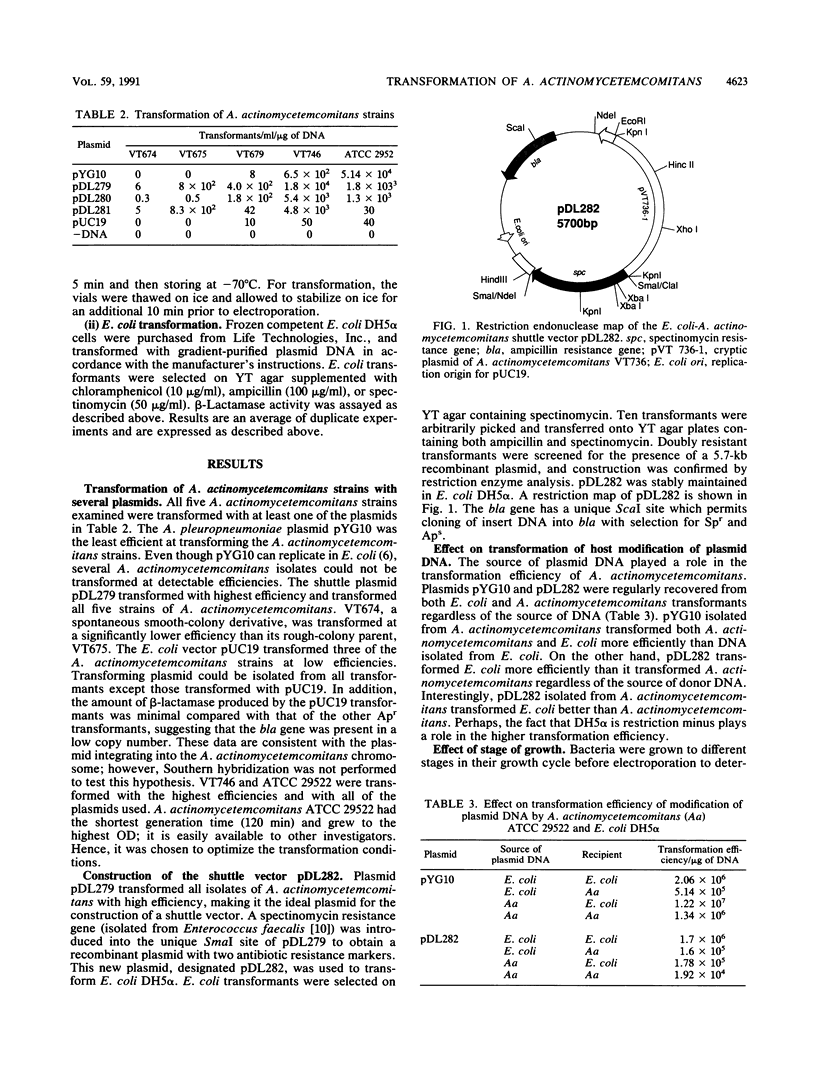
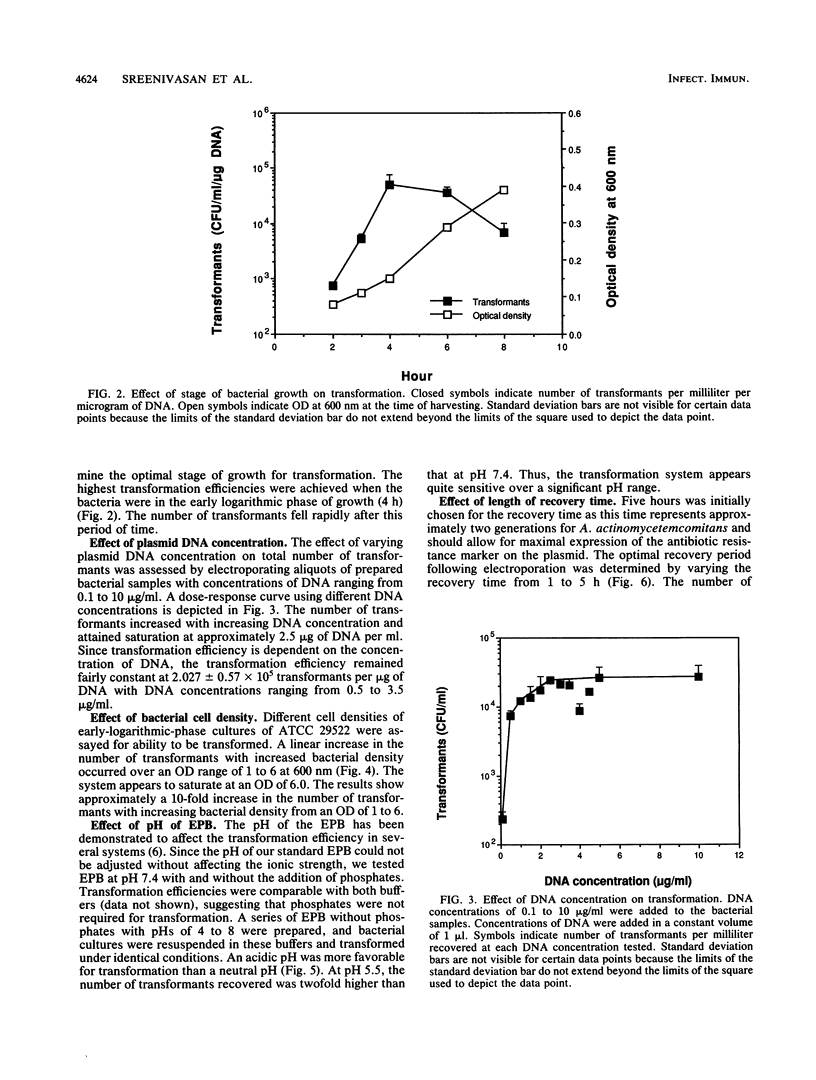
Actinobacillus actinomycetemcomitans, a periodontal pathogen, has been strongly implicated in human periodontal disease. Advances in the molecular analysis of A. actinomycetemcomitans virulence factors have been limited due to the unavailability of systems for genetic transfer, transposon mutagenesis, and gene complementation. Slow progress can be traced almost exclusively to the lack of gene vector systems and methods for the introduction of DNA into A. actinomycetemcomitans. An electrotransformation system that allowed at least five strains of A. actinomycetemcomitans to be transformed with stable shuttle plasmids which efficiently replicated in both Escherichia coli and A. actinomycetemcomitans was developed. One plasmid, a potential shuttle vector designated pDL282, is 5.7 kb in size, has several unique restriction enzyme sites, and codes for resistance to spectinomycin and ampicillin. E. coli and A. actinomycetemcomitans were transformed with equal efficiencies of approximately 10(5) transformants per micrograms of DNA. Similar transformation efficiencies were obtained whether the plasmid DNA was isolated from A. actinomycetemcomitans or E. coli. In addition, frozen competent cells of A. actinomycetemcomitans yielded comparable efficiencies of transformation. Restriction enzyme analysis of pDL282 isolated after transformation confirmed the presence of intact donor plasmids. A plasmid isolated from A. pleuropneumoniae was also capable of transforming some isolates of A. actinomycetemcomitans, although generally at a lower frequency. The availability of these shuttle plasmids and an efficient transformation procedure should significantly facilitate the molecular analysis of virulence factors of A. actinomycetemcomitans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christersson L. A., Albini B., Zambon J. J., Wikesjö U. M., Genco R. J. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J Periodontol. 1987 Aug;58(8):529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Lee L. N., LeBlanc D. J. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991 Apr;57(4):1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Lalonde G., Miller J. F., Tompkins L. S., O'Hanley P. Transformation of Actinobacillus pleuropneumoniae and analysis of R factors by electroporation. Am J Vet Res. 1989 Nov;50(11):1957–1960. [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Inamine J. M. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD(9) determinant from Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Sep;35(9):1804–1810. doi: 10.1128/aac.35.9.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. H., Sreenivasan P. K., Fives-Taylor P. M. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991 Aug;59(8):2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Dower W. J., Tompkins L. S. High-voltage electroporation of bacteria: genetic transformation of Campylobacter jejuni with plasmid DNA. Proc Natl Acad Sci U S A. 1988 Feb;85(3):856–860. doi: 10.1073/pnas.85.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny A., Behling U. H., Hammond B., Lai C. H., Listgarten M., Pham P. H., Sanavi F. Release of toxic microvesicles by Actinobacillus actinomycetemcomitans. Infect Immun. 1982 Jul;37(1):151–154. doi: 10.1128/iai.37.1.151-154.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson P. B., Lantz M., Marucha P. T., Kornman K. S., Trummel C. L., Holt S. C. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982 May;17(3):275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Kushner M. E., Tsai C. C. Inhibition of fibroblast proliferation by Actinobacillus actinomycetemcomitans. Infect Immun. 1982 Dec;38(3):986–992. doi: 10.1128/iai.38.3.986-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. E., Bartholomew E., Genco R. J., Slots J., Levine M. J. Inhibition of neutrophil chemotaxis by soluble bacterial products. J Periodontol. 1982 Aug;53(8):502–508. doi: 10.1902/jop.1982.53.8.502. [DOI] [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]


