Abstract
This review provides a summary of the current state of optical breast imaging and describes its potential future clinical applications in breast cancer imaging. Optical breast imaging is a novel imaging technique that uses near-infrared light to assess the optical properties of breast tissue. In optical breast imaging, two techniques can be distinguished, i.e. optical imaging without contrast agent, which only makes use of intrinsic tissue contrast, and optical imaging with a contrast agent, which uses exogenous fluorescent probes. In this review the basic concepts of optical breast imaging are described, clinical studies on optical imaging without contrast agent are summarized, an outline of preclinical animal studies on optical breast imaging with contrast agents is provided, and, finally, potential applications of optical breast imaging in clinical practice are addressed. Based on the present literature, diagnostic performance of optical breast imaging without contrast agent is expected to be insufficient for clinical application. Development of contrast agents that target specific molecular changes associated with breast cancer formation is the opportunity for clinical success of optical breast imaging.
Keywords: Optical imaging, Breast cancer, Fluorescence, Absorption, Molecular imaging
Background
Breast cancer is a major global health problem. As of 2007, an estimated 1.3 million new cases of invasive breast cancer are diagnosed annually and about 465,000 women are expected to die from this disease worldwide[1]. X-Ray mammography is used in screening programs and reduces mortality significantly due to earlier detection of breast cancer[2,3]. For younger women, the benefit from screening with X-ray mammography is markedly less than for women over the age of 50 years. This is probably caused by the lower incidence of breast cancer at a younger age, the more rapidly growing tumours, and the higher radiographic breast density in young women[4]. Sensitivity of X-ray mammography for breast cancer detection in women with fatty breasts is approximately 88%, but this sensitivity is strongly reduced in women with dense breasts, i.e. 62%[5]. This is an important problem, especially since these women have an increased risk of breast cancer[6].
Optical breast imaging is a novel imaging technique that uses near-infrared (NIR) light to assess the optical properties of tissue, and is expected to play an important role in breast cancer detection. It dates back to 1929 when Cutler investigated the shadows of light transmitted through the breast with a normal lamp (transillumination)[7]. Although large malignant lesions with high vascularization could be detected, the method did not achieve sufficient sensitivity and specificity to be used in clinical practice at the time. During the last decade, progress in source and detector technology, light propagation modelling, and potential fluorescent contrast agents, has resulted in a renewed interest in optical imaging[8]. Optical breast imaging uses near-infrared (NIR) light in the wavelength range of 600–1000 nm to assess the optical properties of tissue. Functional information on tissue components, i.e. absorption characteristics of oxy- and deoxyhaemoglobin, water, and lipid, can be obtained by combining images acquired at various wavelengths. When using only intrinsic breast tissue contrast in optical breast imaging, this is referred to as optical breast imaging without contrast agent. The other modality, i.e. optical breast imaging with a contrast agent, uses exogenous fluorescent probes that target molecules specific for breast cancer. The use of fluorescent probes has great potential in early breast cancer detection, since in vivo imaging of molecular changes associated with breast cancer formation is technically feasible. Additional advantages of optical breast imaging are that it uses no ionizing radiation and it is relatively inexpensive, which can realize repeated use (also in young women) and easy access to the technique. The aim of this review is to provide a summary of the current state of optical breast imaging and to describe its potential future clinical applications in breast cancer imaging.
The basic concepts of optical breast imaging
In general, optical imaging devices transmit light through the breast, where it is both absorbed and scattered by the tissue components present. NIR in the wavelength range of 600–1000 nm is used to allow for sufficient tissue penetration. After passing through the breast, the remaining light is registered by detectors and advanced computer algorithms are used to reconstruct the images (Figs. 1 and 2)[9–11]. Determining tissue properties and their spatial distribution is complex due to the irregular and long pathways over which light travels through the breast[12].
Figure 1.
Prototype of the diffuse optical tomography system used for clinical research (Philips Healthcare, Best, The Netherlands).
Figure 2.
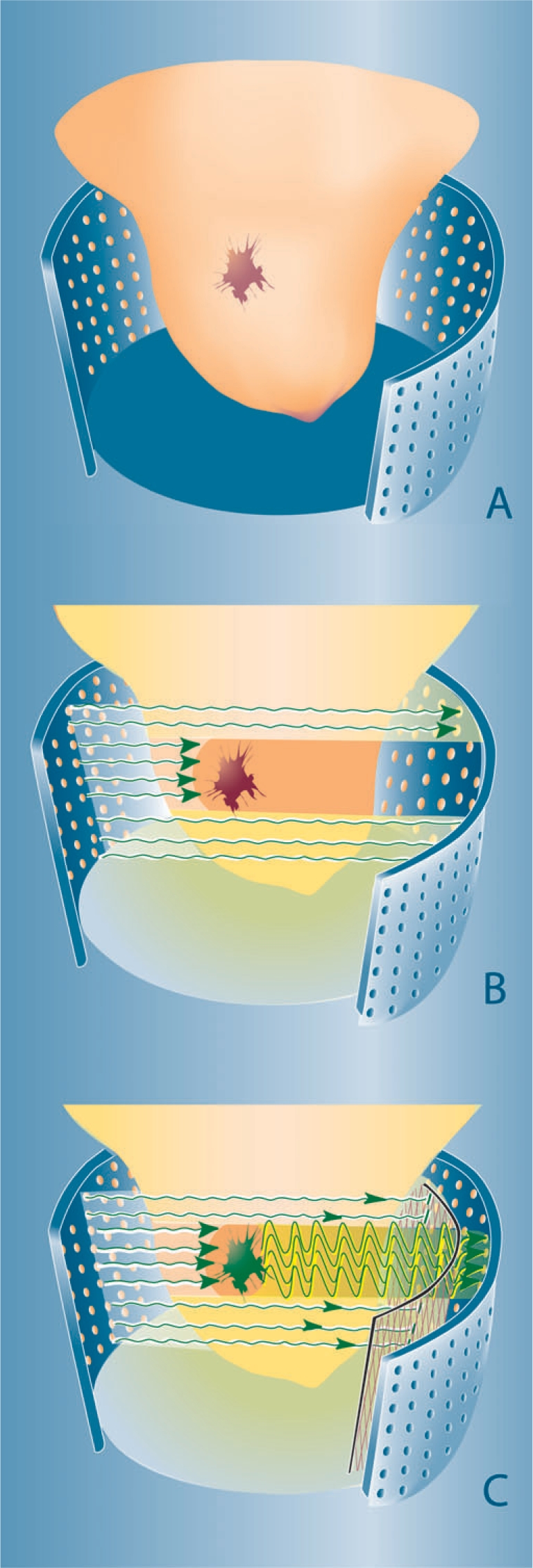
Concepts of optical breast imaging. Optical breast imaging lay-out (A) with source and detector fibres covering the entire breast surface. In optical breast imaging without contrast agent (B) higher absorption by tumour components (predominantly haemoglobin) results in decreased light intensity registered by the detectors. In optical breast imaging with contrast agent (C) a fluorescent probe is administered that ideally accumulates at the tumour site. After excitation, light is emitted at a higher wavelength by this agent and the excitation wavelength is filtered to only detect the fluorescent signal.
Different optical breast imaging systems have been investigated. In transillumination, sources and detectors are positioned at opposite sides of the breast. This generates two-dimensional projection views, comparable to X-ray mammography, and usually requires breast compression[13–17]. In tomography, sources and detectors are placed over the entire breast surface[18,19]. This enables the acquisition of three-dimensional optical breast images. Another approach is the use of handheld devices that are placed manually at the position of interest, comparable to imaging with ultrasound probes[20–22].
Although companies and academic institutions have put vast effort into designing optical breast imaging systems, only three of them are commercially available at this moment. The ComfortScan® system, developed by DOBI Medical, is a transillumination system that requires breast compression to generate two-dimensional optical images (http://www.dobimedical.com/dobisys1.html). SoftScan®, by Advanced Research Technologies Inc. (ART), is a system that uses slight breast compression, but is able to generate tomographic images of a chosen region of interest of the breast. This is the only commercial system that uses more than one laser, namely four, to be able to transmit light of different wavelengths through the breast (http://www.art.ca/en/products/softscan.html)[23]. The Computed Tomography Laser Mammography system CTLM®, developed by Imaging Diagnostic Systems Inc. (IDSI), is a tomographic system that requires no breast compression to generate volumetric optical images of the breast (http://www.imds.com/products/ctlm/).
All optical imaging systems in general, use three different illumination methods: time domain, frequency domain, and continuous wave. The time domain technique uses short (50–400 ps) light pulses to assess the temporal distribution of photons[15,16,24]. In this way, distinction between scattering and absorption can be made. This technology collects the most information on the optical properties of tissue and therefore has better contrast and spatial resolution compared to the other methods. However, time domain equipment is more expensive and acquisition times are longer. Frequency domain devices modulate the amplitude of the light that is continuously transmitted at high frequencies (50–500 MHz)[25]. By measuring phase shifts of photons and their amplitude decay (compared to a reference signal), information on the optical properties of tissue is acquired and scattering and absorption can be distinguished. Frequency domain devices could generate the same information as time domain systems if a large range of frequencies is used[25]. Continuous wave systems emit light at constant intensity or modulated at low frequencies (0.1–100 kHz)[26]. It is a straightforward technique, which basically measures the attenuation of light transmitted between two points on the breast surface. Because of its simplicity, continuous wave equipment is cheap and image acquisition fast. However, it is very difficult to discriminate scattering from absorption with this technique and data analysis requires complex reconstruction algorithms[27].
Optical breast imaging without contrast agent
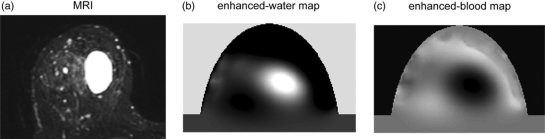
Optical breast imaging uses NIR light to assess the optical properties of breast tissue. Light absorption at these wavelengths is minimal, allowing for sufficient tissue penetration (up to 15 cm). The main components of the breast all have specific absorption characteristics as a function of the wavelength. By combining images acquired at various wavelengths (spectroscopy), concentrations of oxy- and deoxyhaemoglobin, water and lipid can be determined. Fig. 3 demonstrates an example of a benign cyst imaged with both magnetic resonance imaging (MRI) and optical imaging; spectroscopic analysis of the optical data confirmed the high water and low blood concentration in this lesion[28].
Figure 3.
T2-weighted MRI with fat-suppression compared to the enhanced-water map and the enhanced-blood map of the optical data set. The cyst shows a high signal intensity on the MRI and the enhanced-water map (high water content), and a low signal intensity on the enhanced-blood map (low blood content)[28].
In a malignant tumour, haemoglobin concentration is directly related to angiogenesis, the key factor required for tumour growth and metastases[29]. In addition, the proportions of oxy- and deoxyhaemoglobin change in such a tumour due to its metabolism[30]. By measuring the concentrations of the breast components, discrimination of benign and malignant tumours may be possible with diffuse optical imaging (Fig. 2A and B).
Clinical studies thus far performed using optical breast imaging without contrast agent are shown in Table 1[13–24,31–34]. Case reports are not presented in this overview. Most studies report the number of lesions detected on the optical images (detection rates), irrespective of their classification (benign/malignant). Sensitivity and specificity have not been determined yet. Detection rates for carcinomas range from 0.50 to 1.00 in these studies. Studies performed with handheld devices report high detection rates (0.95–1.00)[20–22]. Detection rates for the transillumination approach range from 0.58 to 0.94[13–17]. With tomography, carcinomas were detected in 74%[18] and 50%[19]. Detection rates of benign lesions vary between 0.04 and 0.94[14–21,31]. Benign cysts were detected with tomography in 83%[31]. Malignant lesions were detected by their higher optical attenuation compared to the surrounding tissue, mainly related to increased light absorption by their higher haemoglobin content[20–24,33,34]. Solid benign lesions were more difficult to detect, but sometimes showed increased attenuation, although to a lesser extent than malignant lesions[14–21,31]. As opposed to the other lesions, benign cysts showed lower optical attenuation, associated with lower light absorption or scattering by their high water content[16,24,31]. Some groups found lower oxygenation for carcinomas compared to the surrounding tissue[21,23,24,33]. In addition, Cerussi et al. described increased water content and decreased lipid content in malignant lesions, and age-dependency of the tumour spectra[33]. This group also investigated the response to chemotherapy in breast cancer patients and reported significant decrease in deoxyhaemoglobin (27%) and relative water content (20%) in responders compared to non-responders; oxyhaemoglobin decreased in both groups, but significantly more in responders (33%) compared to non-responders (18%)[32].
Table 1.
Clinical studies on optical breast imaging without contrast agent
| Author (year) | Number of patients | System |
Pre-knowledge on localization | Detection cut-off | Detection rate |
||||
|---|---|---|---|---|---|---|---|---|---|
| Technique | Wavelengths (in nm)a | Imaging approach | Invasive carcinomas† | In situ carcinomas † | Benign lesions | ||||
| Rinneberg et al. (2005)[15] | 159 | Time domain | 670, 785, 843, 884 | Transillumination | Yes. X-Ray and MRI | Weak contrast, tumor only detectable provided exact location of inhomogeneity is known | 0.95 (80/84) | 0.78 (7/9) | 0.89 (39/44) |
| Floery et al. (2005)[18] | 100 | Continuous wave | 808 | Tomography | Yes. X-Ray | Increased absorption; an area clearly more luminous than the surrounding parenchyma | 0.76 (32/42) | 0.38 (3/5) | 0.33 (18/55) |
| Taroni et al. (2005)[16] | 194 | Time domain | 637, 656, 683, 785, 913, 975 | Transillumination | Yes. X-Ray | Weak contrast | 0.89 (50/56) | – | 0.60 (101/169) |
| Yates et al. (2005)[19] | 24 | Time domain | 780, 815 | Tomography | Yes. Different modalities (e.g. MRI, US) | Weak contrast | 0.50 (1/2) | 0.91 (10/11) | |
| Zhu et al. (2005)[20] | 65 | Frequency domain | 780, 830 | Handheld with US guidance | Yes. US | Maximum hemoglobin concentration > 95 μmol/L | 1.00 (8/8) | – | 0.04 (3/73) |
| Götz et al. (1998)[14] | 119 | Frequency domain | 690, 750, 790, 860 | Transillumination | Yes. X-Ray | Clearly visible contrast | 0.86 (51/59) | 0.58 (14/24) | |
| Tomandl et al. (1995)[17] | 102 | Frequency domain | 794, 850 | Transillumination | No | Based on non-specified criteria developed by an experienced radiologist who compared optical images with X-ray and US findings | 0.58 (23/40) | 0.27 (6/22) | |
| Franceschini et al. (1997)[13] | 15 | Frequency domain | 690, 810 | Transillumination | Yes. X-Ray and US | Visible optical inhomogeneity corrected for edge effects (so-called dimensionless N value) | 0.73 (11/15) | – | |
| Chance et al. (2005)[21] | 116 | Continuous wave | 760, 805, 850 | Handheld | Yes. Unspecified | Relatively high hemoglobin content and low oxygenation | 0.95 (42/44) | 0.94 (68/72) | |
| Gu et al. (2004)[31] | 6 | Continuous wave | 785, 808, 830 | Tomography | Yes. X-Ray and US | Lower absorption and/or scattering coefficients than surrounding parenchyma | – | – | 0.83 (5/6) |
| Hsiang et al. (2005)[22] | 6 | Frequency domain | 658, 682, 785, 810, 830, 850 | Handheld | Yes. MRI | Optical index > 2.4 | 1.00 (6/6) | – | – |
| Intes (2005)[23] | 49 | Time domain | 760, 780, 830, 850 | Tomography with breast compression | Yes. X-Ray | Malignant lesions show higher haemoglobin content and lower oxygenation than surrounding parenchyma | |||
| Durduran et al. (2005)[34] | 7 | Continuous wave | 785 | Handheld | Yes. Palpation | Blood flow increases to 230% in malignant lesions and to 153% in benign lesions | |||
| Cerussi et al. (2006)[33] | 57 | Frequency domain + continuous wave | 650–1000 | Handheld | Yes. X-Ray | Malignant lesions show increase in deoxyhaemoglobin, oxyhaemoglobin, and water (>50%), and decrease in lipid (∼20%) compared to normal tissue; tumour spectra appeared age-dependent | |||
| Cerussi et al. (2007)[32] | 11 | Frequency domain + continuous wave | 650–1000 | Handheld | Yes. X-Ray, US, palpation | Responders to chemotherapy showed significant decrease in deoxyhaemoglobin (27%) and relative water content (20%) compared to non-responders; oxyhaemoglobin decreased in both groups, but significantly more in responders (33%) compared to non-responders (18%) | |||
| Ntziachristos et al. (2002)[24] | 14 | Time domain | 780, 830 | Transillumination combined with MRI | Yes. Concurrent MRI | Malignant lesions show higher haemoglobin content and lower oxygenation than surrounding parenchyma | |||
US, ultrasound; MRI, magnetic resonance imaging.
aWavelengths in bold were used in all measurements, others only in part of the measurements.
bWhen there were less than 5% in situ carcinomas, this group was combined with invasive carcinomas (italic), and also when there was no information on invasiveness available.
Optical breast imaging with contrast agent
In optical breast imaging with contrast agent, fluorescent probes are used that emit photons at predefined wavelengths after excitation by laser light. These photons are detected while the light of the excitation wavelength is filtered (Fig. 2C).
Fluorescent probes that target molecules specific for breast cancer are currently being developed and validated in preclinical animal studies. An overview of these studies is provided in Table 2[35–43]. All animal studies were performed with breast cancer mouse models with NIR continuous wave optical imaging devices. In most studies, transillumination was used, but two research groups applied a tomographic approach[35,41]. A variety of optical probes for specific breast cancer cell targeting has been designed. The group of Bremer and Mahmood et al. developed so-called ‘smart’ optical probes to target proteases[35–37]. These probes are non-fluorescent in their native state, but convert to a highly fluorescent active state when their backbone is cleaved by cathepsins. In four animals with human breast cancer xenografts, tumours showed a strong fluorescence signal in vivo after injection of the cathepsin-sensing probe. Signal-to-noise ratio (SNR) after 48 h was 21 in tumours with mean diameters <2 mm. The smallest detectable tumour was <1 mm in diameter[37]. This technique using smart optical probes also showed good results in transgenic mice that spontaneously developed tumours. With transillumination, all 24 tumours in 10 animals could be clearly delineated after injection of the cathepsin-sensing probe. Tumour fluorescence in vivo was significantly higher compared to background fluorescence measured in the adjacent skin (380 ± 23 AU vs. 179 ± 8 AU; p < 0.01). Tomography was performed in four animals; co-registration with MRI revealed a strong fluorescence signal within the tumour tissue and virtually no background fluorescence in the corresponding slices[35]. Differences in tumour aggressiveness could be depicted by this technique when comparing eight well-differentiated, with eight highly invasive metastatic human breast cancer models. The highly aggressive cancers, which expressed higher levels of proteases, revealed significantly higher tumour fluorescence compared to well-differentiated tumours (861 ± 88 AU vs. 566 ± 36 AU; p < 0.01). Tumours in non-injected animals were not visible due to identical autofluorescence in tumour and adjacent skin[36].
Table 2.
Preclinical studies on optical breast imaging with contrast agent
| Author (year) | Subjects (n) | System |
Use of other modality | Target | Optical imaging probe | Injection | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Technique | Wavelengths (nm) excitation/emission | Imaging approach | |||||||
| Bremer et al. (2005)[35] | Transgenic mice, spontaneously developing breast cancer (10) | Continuous wave | 610–650/680–720; tomography: 670 | Transillumination and tomography (n = 4) | MRI | Cathepsin-B (protease) | Cathepsin-sensing probe with Cy5.5 fluorochrome residues bound to a poly-lysine backbone sterically shielded through MPEG side chains, activated by enzymatic cleavage of the backbone | Intravenous 2 nmol | Strong fluorescence signal within tumour tissue with virtually no background fluorescence in corresponding slices |
| Wang et al. (2007)[44] | Mice bearing human breast cancer xenograft in thigh | Continuous wave | 785 | Transillumination | SPECT/CT | Interleukin-11 receptor alpha-chain | Dual-labelled probe consisting of a cyclic nonapeptide as targeting component, an 111In complex as radiotracer, and an NIR dye as optical signal generator | Intravenous 2 nmol | Both optical imaging and SPECT/CT show high uptake of probe at the tumour site |
| Mahmood et al. (1999)[37] | Mice bearing human breast cancer xenograft in mammary fat pad or thigh (4) | Continuous wave | 610–650/680–720 | Transillumination | – | Cathepsins B and H (proteases) | Ezyme-activatable probe with Cy5.5 fluorochrome residues bound to a poly-lysine backbone sterically shielded through MPEG side chains, activated by enzymatic cleavage of the backbone | Intravenous 10 nmol | Strong fluorescence signal within tumour tissue, detection of tumour <1 mm |
| Bremer et al. (2002)[36] | Mice bearing aggressive (8) or non-aggressive (8) human breast cancer xenograft in mammary fat pad | Continuous wave | 610–650/680–720 | Transillumination | – | Cathepsin-B (protease) | Cathepsin-sensing probe with Cy5.5 fluorochrome residues bound to a poly-lysine backbone sterically shielded through MPEG side chains, activated by enzymatic cleavage of the backbone | Intravenous 2 nmol | Clearly visible fluorescence signal in all tumours; aggressive tumours (with stronger cathepsin-B expression) showed significantly higher fluorescence values than non-aggressive tumours |
| Sampath et al. (2007)[42] | Mice bearing HER2-overexpressing human breast cancer xenograft in thigh (18) | Continuous wave | 785/830 | Transillumination | SPECT/CT | HER2 | Dual-labelled probe consisting of trastuzumab (monoclonal antibody) as targeting component, an 111In complex as radiotracer, and an NIR dye as optical signal generator | Intravenous 0.43 nmol | Strong fluorescence signal at tumour site, uptake significantly higher compared to non-specific probes and to mice pretreated with trastuzumab; SPECT/CT showed similar patterns in probe uptake |
| Ke et al. (2003)[40] | Mice bearing EGF receptor-positive/negative human breast cancer xenograft in mammary fat pad | Continuous wave | 660/710 | Transillumination | – | EGF receptor | EGF-Cy5.5 conjugate | Intravenous 1 nmol | Clearly visible fluorescence signal in EGF receptor-positive tumours, no uptake in EGF receptor-negative tumours, antibody C225 specifically blocked uptake |
| Hilger et al. (2004)[39] | Mice bearing human breast cancer xenograft in thigh, with or without HER2-overexpression (6) | Continuous wave | 675/708 | Transillumination | – | HER2 | Herceptin (monoclonal antibody) coupled to Cy5.5 | Intravenous | Distinct fluorescence signal in HER2-overexpressing tumours compared to normal expressing tumours |
| Montet et al. (2005)[41] | Mice bearing HER2-overexpressing human breast cancer xenograft in mammary fat pad (5) | Continuous wave | 672, 748 | Tomography | MRI and SPECT/CT | Angiogenesis and HER2 | Angiosense-750 (an NIR fluorochrome labelled vascular marker), and Herceptin (monoclonal antibody) coupled to Cy5.5 | Intravenous co injection | Significant fluorescence signal at tumour site for both the HER2 and the angiogenesis targeting probe |
| Yang et al. (2007)[45] | Mice bearing human breast cancer xenograft in forepaw | Continuous wave | 730/790 | Transillumination | X-Ray | Non-specific tumor accumulation | Core-cross-linked polymeric micelles (CCPMs) conjugated with Cy7-like NIR dye (intravenous injection 4.5 nmol) | Intravenous | Strong fluorescence signal at tumour site |
MPEG, methoxypolyethylene glycol; NIR, near-infrared; MRI, magnetic resonance imaging; SPECT, single photon emission computed tomography; CT, computed tomography; HER, human epidermal growth factor receptor; EGF, epidermal growth factor; ICG, Indocyanine Green.
Three research groups focused on targeting the human epidermal growth factor-2 (HER2) receptor with probes containing the humanized monoclonal anti-HER2 antibody trastuzumab, Herceptin, coupled to an NIR dye[39,41,42]. Hilger et al. compared such probes in three animals with HER2-overexpressing tumours and three animals with normal HER2-expression. Distinctly higher relative fluorescence signals were found in the tumours with HER2-overexpression compared to the tumours with normal HER2-expression (e.g. 16 h after injection: 2.2 ± 0.1 vs. 1.3 ± 0.2)[39]. Sampath and colleagues designed a dual-labelled probe consisting of trastuzumab as targeting component, an 111In complex as radiotracer, and an NIR dye as optical signal generator. Fluorescence signal intensities obtained after injection with this HER2-specific probe in three mice bearing HER2-overexpressing tumours, were significantly higher (tumour-to-muscle ratio (TMR) 2.25 ± 0.2) compared to fluorescence signal intensities after injection of two non-specific probes (TMR 1.35 ± 0.1 and 1.44 ± 0.18; p ≤ 0.001), each administered in five mice. TMR in five mice pre-injected with trastuzumab before receiving the HER2-specific probe was significantly lower than in the mice not pre-injected (p = 0.0048). Single photon emission computed tomography (SPECT) fused with computed tomography (CT) showed similar patterns in probe uptake[42]. Montet et al. co injected two optical probes, an NIR fluorochrome labelled vascular marker (Angiosense-750) and Herceptin coupled to an NIR dye, at the same time in an HER2-overexpressing breast cancer mouse model. This model showed significant tumoural uptake of both the vascular marker (3.1 ± 0.5%) and the HER2-specific probe (14.7 ± 1.3 pmol), indicating that studying two different physiologic variables simultaneously is feasible with this technique[41].
Ke et al. used human epidermal growth factor (EGF) coupled to an NIR dye to detect the EGF receptor in breast cancer cells. A fluorescence signal was clearly visualized in EGF receptor-positive tumours but not in EGF receptor-negative tumours. The uptake of the probe was blocked by the anti-EGF receptor antibody C225, indicating specificity of the probe for the EGF receptor[40]. Another target for breast cancer detection with fluorescent probes is the Interleukin-11 receptor alpha-chain, investigated by Wang et al. They designed a dual-labelled probe consisting of a cyclic nonapeptide as targeting component, an 111In complex as radiotracer, and an NIR dye as optical signal generator. Both optical imaging and SPECT/CT showed high uptake of this probe at the tumour site in mice[44].
Non-specific tumour accumulation through polymeric micelles was studied by Yang et al. These micelles remained in the circulation for a prolonged time and effectively accumulated at the tumour site through microvascular hyperpermeability, displaying a strong fluorescence signal[45].
Aforementioned studies demonstrate proof of principle of optical breast imaging with fluorescent probes in an animal model. Thus far, only two clinical optical breast imaging studies with contrast agent have been described in the literature, both using Indocyanine Green (ICG) in 3 patients[46,47]. ICG is a non-specific blood pool agent that is both absorbing and fluorescent in the NIR range. It is used clinically, mainly for retinal angiography and liver function tests. Both studies observed differences in ICG pharmacokinetics between malignant and benign lesions on the optical images. In the study by Intes et al. a maximal increase in absorption of 0.042 cm−1 was measured in an invasive ductal carcinoma. For an adenoma, an absorption increase of 0.025 cm−1 was found. The absorption increase observed in a fibroadenoma was ∼0.03 cm−1[46]. In a patient with invasive ductal carcinoma Ntziachristos et al. found an increase in absorption at the tumour position of ∼0.05 cm−1. The increase in absorption in a fibroadenoma was ∼0.03 cm−1. In healthy tissue some moderate enhancements were seen of ∼0.025 cm−1[47].
Discussion
Clinical studies performed on optical breast imaging without contrast agent showed cancer detection rates ranging from 0.50 to 1.00 for different optical imaging systems. Since knowledge on lesion localization within the breast was available in almost all studies, true detection rates will probably be substantially lower. Information available from the literature is too scarce to determine sensitivity and specificity of optical breast imaging without the use of contrast agent for breast cancer detection. Although differences were found in haemoglobin and oxygenation between carcinomas and benign lesions, the sensitivity and specificity achieved by optical breast imaging without contrast agent seem currently insufficient to use this modality in clinical practice. In a study setting, optical breast imaging without contrast agent is presently being explored to evaluate the response to neoadjuvant chemotherapy in patients with a known breast cancer. These patients have large tumours the position of which is known a priori. Biochemical changes in tumour tissue often precede anatomical alterations (e.g. tumour shrinkage) after chemotherapy. Optical breast imaging can thus potentially be applied to predict the response to neoadjuvant chemotherapy earlier in the treatment cycle.
Preclinical studies showed that probes designed to target specific proteins characteristic for breast cancer can successfully detect breast tumours using optical imaging in animal models. Optical imaging probes have been developed to target the following proteins: Cathepsin B and H, HER2, EGF receptor, and Interleukin-11 receptor alpha-chain. Blood pool agents without a specific target, such as Angiosense-750, ICG, and polymeric micelles, have also been assessed to visualize tumours and their associated angiogenesis. With the exception of ICG, the optical probes have not yet been tested in humans. In breast cancer mouse models, optical imaging with contrast agent showed very promising results. A strong fluorescence signal was obtained from tumour tissue in comparison to tissue that did not overexpress the target of interest. Most studies confirmed their results with histology and/or SPECT/CT imaging of probe uptake.
As mentioned before, breast imaging modalities in current use have some limitations. The sensitivity of X-ray mammography for breast cancer detection is reduced in women with dense breasts (62%)[5]. This issue is especially important since these women have an increased risk of breast cancer[6]. Breast MRI has high sensitivity (>95%) and is currently used in clinical practice as an adjunct to X-ray mammography for screening of high risk patients[48,49]. Despite substantial improvements in imaging technology, MRI in general only allows lesion detection and classification when tumour size is 5 mm or more[50]. As lesion size upon discovery decreases with more efficient screening programs, the need for a non-invasive tool that provides more specific information on small breast lesions becomes obvious.
Optical breast imaging could be the modality with this potential, because of its molecular imaging capability. Molecular imaging is defined as the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems[51]. A major advantage is that molecular changes associated with cancer formation may possibly be detected at a very early stage, even before anatomical changes occur. With the use of target-specific probes, optical imaging could be a valid candidate for the early detection of breast cancer, e.g. in young women with dense breasts. Other potential applications of this technique may be the selection of appropriate treatment and evaluation of response to treatment in breast cancer patients. If the molecular characteristics of breast tumours can be identified in vivo using optical breast imaging with contrast agents, this molecular tumour profile can be used to select appropriate therapies for individual patients (personalized medicine). Moreover, response to therapy can be evaluated using the same imaging technique.
Important advantages of optical imaging with contrast agent are that it does not use any radioactive components (as in positron emission tomography (PET) and SPECT), and that its sensitivity for probe detection is very high (possibly in the nanomolar to the 100 picomolar concentration range) as compared to MRI (micromolar to millimolar range). Moreover, optical imaging uses no ionizing radiation and can thus be used repeatedly, also in younger women. Non-toxic fluorescent probes that can be applicable in clinical practice are currently being developed. At present, a single molecular marker that is expressed by all different types of breast cancers is not available. Likely, a combination of fluorescent probes would have to be administered to be able to detect all breast cancer types with this technique. The use of a single probe targeting one of the breast cancer types could nevertheless be valuable to select patients for, and monitor certain cancer treatments. The need to inject one or more contrast agent(s) intravenously prior to the optical imaging studies may comprise some practical limitations. For instance, interactions between the probes need to be thoroughly investigated, as well as the optimal imaging time points after their injection. Another limitation of optical breast imaging is its low spatial resolution. Spatial resolution in optical imaging is dependent on the length of the light pathways, resulting in lower spatial resolution when tissue penetration is deeper. If detailed anatomical information is also needed, a second imaging technique or a multi-modality imaging approach in which the optical scanner is combined with another modality (e.g. MRI, ultrasound) could offer a solution.
In conclusion, the diagnostic performance of optical breast imaging without contrast agent is likely inadequate for clinical application. Development of contrast agents that target specific molecular changes associated with breast cancer formation is the opportunity for clinical success of optical breast imaging.
References
- 1.Garcia M, Jemal A, Ward EM, et al. Atlanta, GA: American Cancer Society; 2007. Global cancer facts & figures 2007. [Google Scholar]
- 2.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–80. doi: 10.1056/NEJMcp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137:347–60. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 4.Buist DS, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40–49 years. J Natl Cancer Inst. 2004;96:1432–40. doi: 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- 5.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 6.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 7.Cutler M. Transillumination as an aid in the diagnosis of breast lesions. Surg Gynecol Obstet. 1929;48:721–8. [Google Scholar]
- 8.Gibson AP, Hebden JC, Arridge SR. Recent advances in diffuse optical imaging. Phys Med Biol. 2005;50:R1–43. doi: 10.1088/0031-9155/50/4/r01. [DOI] [PubMed] [Google Scholar]
- 9.Arridge SR, Schweiger M. Image reconstruction in optical tomography. Philos Trans R Soc Lond B Biol Sci. 1997;352:717–26. doi: 10.1098/rstb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweiger M, Arridge SR, Nissila I. Gauss–Newton method for image reconstruction in diffuse optical tomography. Phys Med Biol. 2005;50:2365–86. doi: 10.1088/0031-9155/50/10/013. [DOI] [PubMed] [Google Scholar]
- 11.Schweiger M, Nissila I, Boas DA, Arridge SR. Image reconstruction in optical tomography in the presence of coupling errors. Appl Opt. 2007;46:2743–56. doi: 10.1364/ao.46.002743. [DOI] [PubMed] [Google Scholar]
- 12.Arridge SR, Hiraoka M, Schweiger M. Statistical basis for the determination of optical pathlength in tissue. Phys Med Biol. 1995;40:1539–58. doi: 10.1088/0031-9155/40/9/011. [DOI] [PubMed] [Google Scholar]
- 13.Franceschini MA, Moesta KT, Fantini S, et al. Frequency-domain techniques enhance optical mammography: initial clinical results. Proc Natl Acad Sci U S A. 1997;94:6468–73. doi: 10.1073/pnas.94.12.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Götz L, Heywang-Kobrunner SH, Schutz O, Siebold H. [Optical mammography in preoperative patients] Akt Radiol. 1998;8:31–3. [PubMed] [Google Scholar]
- 15.Rinneberg H, Grosenick D, Moesta KT, et al. Scanning time-domain optical mammography: detection and characterization of breast tumors in vivo. Technol Cancer Res Treat. 2005;4:483–96. doi: 10.1177/153303460500400503. [DOI] [PubMed] [Google Scholar]
- 16.Taroni P, Torricelli A, Spinelli L, et al. Time-resolved optical mammography between 637 and 985 nm: clinical study on the detection and identification of breast lesions. Phys Med Biol. 2005;50:2469–88. doi: 10.1088/0031-9155/50/11/003. [DOI] [PubMed] [Google Scholar]
- 17.Tomandl B, Doinghaus K, Schulz-Wendtland R. [Laser mammography with near-infrared light] Rontgenpraxis. 1995;48:197–201. [PubMed] [Google Scholar]
- 18.Floery D, Helbich TH, Riedl CC, et al. Characterization of benign and malignant breast lesions with computed tomography laser mammography (CTLM): initial experience. Invest Radiol. 2005;40:328–35. doi: 10.1097/01.rli.0000164487.60548.28. [DOI] [PubMed] [Google Scholar]
- 19.Yates T, Hebden JC, Gibson A, Everdell N, Arridge SR, Douek M. Optical tomography of the breast using a multi-channel time-resolved imager. Phys Med Biol. 2005;50:2503–17. doi: 10.1088/0031-9155/50/11/005. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Q, Cronin EB, Currier AA, et al. Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction. Radiology. 2005;237:57–66. doi: 10.1148/radiol.2371041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chance B, Nioka S, Zhang J, et al. Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: a six-year, two-site study. Acad Radiol. 2005;12:925–33. doi: 10.1016/j.acra.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Hsiang D, Shah N, Yu H, et al. Coregistration of dynamic contrast enhanced MRI and broadband diffuse optical spectroscopy for characterizing breast cancer. Technol Cancer Res Treat. 2005;4:549–58. doi: 10.1177/153303460500400508. [DOI] [PubMed] [Google Scholar]
- 23.Intes X. Time-domain optical mammography SoftScan: initial results. Acad Radiol. 2005;12:934–47. doi: 10.1016/j.acra.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Ntziachristos V, Yodh AG, Schnall MD, Chance B. MRI-guided diffuse optical spectroscopy of malignant and benign breast lesions. Neoplasia. 2002;4:347–54. doi: 10.1038/sj.neo.7900244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nissila I, Hebden JC, Jennions D, et al. Comparison between a time-domain and a frequency-domain system for optical tomography. J Biomed Opt. 2006;11:064015. doi: 10.1117/1.2400700. [DOI] [PubMed] [Google Scholar]
- 26.Siegel A, Marota JJ, Boas D. Design and evaluation of a continuous-wave diffuse optical tomography system. Opt Express. 1999;4:287–98. doi: 10.1364/oe.4.000287. [DOI] [PubMed] [Google Scholar]
- 27.Arridge SR, Lionheart WR. Nonuniqueness in diffusion-based optical tomography. Opt Lett. 1998;23:882–4. doi: 10.1364/ol.23.000882. [DOI] [PubMed] [Google Scholar]
- 28.van de Ven SM, Wiethoff AJ, van der Voort M, et al. Spectroscopic diffuse optical imaging of the breast: first clinical experiences in the characterization of cysts. Joint Molecular Imaging Conference 2007, Providence, RI. Academy of Molecular Imaging & Society for Molecular Imaging. [Google Scholar]
- 29.Rice A, Quinn CM. Angiogenesis, thrombospondin, and ductal carcinoma in situ of the breast. J Clin Pathol. 2002;55:569–74. doi: 10.1136/jcp.55.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9:4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 31.Gu X, Zhang Q, Bartlett M, Schutz L, Fajardo LL, Jiang H. Differentiation of cysts from solid tumors in the breast with diffuse optical tomography. Acad Radiol. 2004;11:53–60. doi: 10.1016/s1076-6332(03)00562-2. [DOI] [PubMed] [Google Scholar]
- 32.Cerussi A, Hsiang D, Shah N, et al. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci U S A. 2007;104:4014–19. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerussi A, Shah N, Hsiang D, Durkin A, Butler J, Tromberg BJ. In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J Biomed Opt. 2006;11:044005. doi: 10.1117/1.2337546. [DOI] [PubMed] [Google Scholar]
- 34.Durduran T, Choe R, Yu G, et al. Diffuse optical measurement of blood flow in breast tumors. Opt Lett. 2005;30:2915–17. doi: 10.1364/ol.30.002915. [DOI] [PubMed] [Google Scholar]
- 35.Bremer C, Ntziachristos V, Weitkamp B, Theilmeier G, Heindel W, Weissleder R. Optical imaging of spontaneous breast tumors using protease sensing ‘smart’ optical probes. Invest Radiol. 2005;40:321–7. doi: 10.1097/01.rli.0000163797.23172.90. [DOI] [PubMed] [Google Scholar]
- 36.Bremer C, Tung CH, Bogdanov Jr A, Weissleder R. Imaging of differential protease expression in breast cancers for detection of aggressive tumor phenotypes. Radiology. 2002;222:814–18. doi: 10.1148/radiol.2223010812. [DOI] [PubMed] [Google Scholar]
- 37.Mahmood U, Tung CH, Bogdanov Jr A, Weissleder R. Near-infrared optical imaging of protease activity for tumor detection. Radiology. 1999;213:866–70. doi: 10.1148/radiology.213.3.r99dc14866. [DOI] [PubMed] [Google Scholar]
- 38.Wang LV. Optical tomography for biomedical applications. IEEE Eng Med Biol Mag. 1998;17:45–6. doi: 10.1109/51.664029. [DOI] [PubMed] [Google Scholar]
- 39.Hilger I, Leistner Y, Berndt A, et al. Near-infrared fluorescence imaging of HER-2 protein over-expression in tumour cells. Eur Radiol. 2004;14:1124–9. doi: 10.1007/s00330-004-2257-9. [DOI] [PubMed] [Google Scholar]
- 40.Ke S, Wen X, Gurfinkel M, et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63:7870–5. [PubMed] [Google Scholar]
- 41.Montet X, Ntziachristos V, Grimm J, Weissleder R. Tomographic fluorescence mapping of tumor targets. Cancer Res. 2005;65:6330–6. doi: 10.1158/0008-5472.CAN-05-0382. [DOI] [PubMed] [Google Scholar]
- 42.Sampath L, Kwon S, Ke S, et al. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007;48:1501–10. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Baranov E, Li XM, et al. Whole-body and intravital optical imaging of angiogenesis in orthotopically implanted tumors. Proc Natl Acad Sci U S A. 2001;98:2616–21. doi: 10.1073/pnas.051626698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Ke S, Kwon S, et al. A new optical and nuclear dual-labeled imaging agent targeting interleukin 11 receptor alpha-chain. Bioconjug Chem. 2007;18:397–402. doi: 10.1021/bc0602679. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Zheng S, Harrison WJ, et al. Long-circulating near-infrared fluorescence core-cross-linked polymeric micelles: synthesis, characterization, and dual nuclear/optical imaging. Biomacromolecules. 2007;8:3422–8. doi: 10.1021/bm7005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Intes X, Ripoll J, Chen Y, Nioka S, Yodh AG, Chance B. In vivo continuous-wave optical breast imaging enhanced with Indocyanine Green. Med Phys. 2003;30:1039–47. doi: 10.1118/1.1573791. [DOI] [PubMed] [Google Scholar]
- 47.Ntziachristos V, Yodh AG, Schnall M, Chance B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc Natl Acad Sci U S A. 2000;97:2767–72. doi: 10.1073/pnas.040570597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–8. doi: 10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 49.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 50.Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter? Positive predictive value of MRI-detected breast lesions as a function of lesion size. AJR Am J Roentgenol. 2006;186:426–30. doi: 10.2214/AJR.04.1707. [DOI] [PubMed] [Google Scholar]
- 51.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18N, 21N. [PubMed] [Google Scholar]