Abstract
Basic and clinical observations suggest that thrombosis and inflammation are closely related. Here we addressed the role played by TNF-α in thrombus formation and growth in an in vivo mouse model. Using intravital microscopy, we show that systemic administration of TNF-α at doses found in sepsis transiently inhibits thrombus formation and delays arterial occlusion upon vascular injury. These results were reflected in a prolonged bleeding time. Platelets isolated from the TNF-α–treated mice showed a marked decrease in fibrinogen binding and P-selectin expression as well as reduced platelet aggregation in response to various agonists. In contrast, in vitro treatment of platelets with TNF-α did not affect their function. TNF receptor 1– and 2–deficient mice exhibited normal thrombogenesis in the presence of TNF-α. Additionally, the inhibitory effect of TNF-α was lost either after treatment with NG-monomethyl-L-arginine, an inhibitor of NO production, or in mice deficient for iNOS. These results indicate that under inflammatory conditions, when leukocytes need free passage to transmigrate into tissues, TNF-α decreases platelet activation and inhibits thrombi formation. This effect is not exerted directly on platelets but mediated through the rapid generation of NO in the vessel wall.
Introduction
Inflammation is often considered a contributing factor to thrombotic and hemostatic disorders associated with various disease states. These diseases include the coagulopathy of septicemia (1), the prothrombotic state associated with atherosclerotic vessels (2) or cancer (3), and the veno-occlusive disease of the liver after bone marrow transplantation (4). Previous studies have shown that the proinflammatory mediators, especially TNF-α, can induce a procoagulant state by eliciting tissue factor production on the surface of vascular endothelium and monocytes, downregulating the protein C anticoagulant pathway and stimulating thrombin and fibrin formation (5). Neither these studies nor those testing the local administration of high doses of TNF-α as a potential cure for cancer patients provided evidence that TNF-α triggers a thrombotic response in vivo (6), however. Although TNF-α is considered to be a key factor involved in the pathogenesis of diseases affecting the cardiovascular system, its role in platelet thrombus formation still remains to be addressed.
TNF receptor-1 and -2 (TNF-R1 and -R2) are found on almost all nucleated cell types but are poorly detectable on platelets. Although it appears that platelets bind TNF-α (7) and that murine megakaryocytes express TNF-R1 (8), at present it is not clear whether normal human platelets express TNF-Rs. In addition, it was recently shown that CD40 ligand (CD40L), a member of the TNF family of ligands, plays a role in the stability of arterial thrombi through the specific binding of its amino acids Lys-Gly-Asp (KGD) KGD sequence to the platelet β3 integrin (9). Interestingly, TNF-α also contains a KGD sequence, but its involvement in platelet thrombus formation has not been investigated.
In this study, we used intravital microscopy to examine the role played by TNF-α in the dynamic high-shear environment of an in vivo model of arterial injury. We show that the systemic administration of TNF-α at a dose described as procoagulant led to a marked defect in thrombus formation and delayed or prevented vessel occlusion. We show that the inhibitory effect of TNF-α is mediated through TNF-Rs and the rapid production of NO.
Methods
Reagents.
Murine recombinant TNF-α and FITC-conjugated rat anti-mouse P-selectin Ab were purchased from PharMingen (San Diego, California, USA). Polyclonal rabbit anti-human fibrinogen antiserum was obtained from DAKO Corp. (Carpinteria, California, USA) and coupled to FITC in our laboratory as described previously (10). Phycoerythrin-conjugated rat anti-mouse αIIbβ3 mAb (JONI-PE) was a gift from B. Nieswandt (Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany). Bovine thrombin and NG-monomethyl-L-arginine (L-NMMA) were purchased from Calbiochem (San Diego, California, USA), and ADP was obtained from Bio/Data Corp. (Horsham, Pennsylvania, USA). Collagen-related peptide (CRP), a gift from J. Hartwig (Brigham and Women’s Hospital, Boston, Massachusetts, USA), was multimerized and characterized as described (11).
Experimental animals.
WT, TNF-R1/2–/– (12), and iNOS–/– (13) mice, all on C57Bl/6J/129Sv background, and WT C57Bl/6J mice were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). Animals were housed at the CBR Institute for Biomedical Research, and experimental procedures were approved by its Animal Care and Use Committee.
Platelet preparation.
Mice were bled from the retro-orbital plexus under isoflurane anesthesia. The blood was collected in a tube containing 7.5 U/ml heparin. Platelet-rich plasma (PRP) was obtained by centrifugation at 300 g for 5 minutes at room temperature. PRP was centrifuged at 1,000 g in the presence of prostacyclin (1 μM) for 5 minutes at room temperature. After two washing steps, pelleted platelets were resuspended in modified Tyrode’s-HEPES buffer (137 mM NaCl, 0.3 mM Na2HPO4, 2 mM KCl, 12 mM NaHCO3, 5 mM HEPES, 5 mM glucose, pH 7.3) containing 0.35% BSA and incubated at 37°C until used.
Intravital microscopy.
Platelets were isolated from PRP and fluorescently labeled with calcein as described (14). Fluorescently labeled platelets (5 × 109 platelets/kg of body weight), to which TNF-α (1 ng/ml or 100 pg/ml of estimated blood volume), L-NMMA (100 μM/15 g of mouse body weight), or Tyrode’s buffer was added, were injected into the retro-orbital plexus of male 3- to 4-week-old mice of matching genotypes. Blood volume was estimated as one-thirteenth of body weight. Animals were anesthetized with 2.5% tribromoethanol (0.15 ml/10 g body weight) and prepared for intravital microscopy of the mesentery as described previously (15). For each recorded mesenteric arteriole (70–100 μm diameter), the centerline erythrocyte velocity and the arterial shear rate were determined as described previously (14) before inducing the injury with ferric chloride (30 μl of a 250-mM solution). Each injured arteriole was recorded until final occlusion or for a 40-minute observation period if occlusion was impaired, with 40 minutes being chosen as the occlusion time.
Bleeding time measurement.
A 3-mm segment of the tail was amputated from untreated and TNF-α–treated WT mice (12–16 weeks old) (16). The tail was immersed in 0.9% isotonic saline at 37°C, and the time required for the stream of blood to stop was defined as the bleeding time.
Plasma clotting time assay and microparticle determination.
One milliliter of blood was drawn from the retro-orbital venous plexus by using plain microhematocrit capillary tubes (VWR, West Chester, Pennsylvania, USA) and collected into tubes containing 10% final volume of acid-citrate-dextrose (17). Platelet-poor plasma (PPP) was prepared by centrifugation at 1,500 g for 25 minutes and then at 10,000 g for 5 minutes to remove contaminating cells. Plasma clotting time was measured in an aggregometer (Sienco Inc., Wheat Ridge, Colorado, USA) as described (17). Microparticles were isolated from PPP and analyzed by flow cytometry as described (17).
Flow-cytometry analysis.
TNF-α was either systematically administered into mice for 30 minutes or added to whole blood or platelet samples for the same length of time in vitro. Washed platelets obtained from either approach were resuspended in modified Tyrode’s buffer containing 1 mM CaCl2 and stimulated with 0.1 U/ml thrombin or 2 μg/ml CRP. Incubation with 5 μg/ml of FITC-conjugated rat anti-mouse P-selectin, FITC-conjugated rabbit anti-human fibrinogen, or the respective FITC-conjugated isotype-matched control antibodies was performed for 10 minutes at 37°C, and the samples were analyzed immediately. For all measurements, a FACScalibur flow cytometer (BD Biosciences, San Jose, California, USA) was used.
Aggregometry.
PRP was obtained as described for platelet preparation. The platelet count was adjusted to 5 × 108 platelets/ml with autologous PPP, and aggregation was induced at 1,000 rpm by 2 μM ADP. Aggregation was measured as light transmission on a four-channel aggregometer (Chrono-Log Corp., Havertown, Pennsylvania, USA). Light transmission was set at 0% for PRP and 100% for PPP. The results were expressed as the maximum percentage of aggregation.
Determination of cGMP levels.
PRP was obtained from control or TNF-α–treated WT mice. Platelets were isolated from PRP, treated for 10 minutes with 0.1 M HCl, and then centrifuged at 600 g at room temperature. The supernatants were analyzed using the cGMP Enzyme Immunoassay Kit according to the manufacturer’s instructions (Biomol Research Laboratories, Plymouth Meeting, Pennsylvania, USA).
Platelet count determination.
Blood was collected in 2.5 mM EDTA from the retro-orbital plexus and diluted using the Unopette microcollection system according to the manufacturer’s instructions (Becton Dickinson, Franklin Lakes, New Jersey, USA). Platelet counts were performed using a hemocytometer.
Statistics.
Data are presented as mean plus or minus SEM. Statistical analysis was performed using the unpaired Student t test. P values less than 0.05 were considered significant.
Results
Effect of TNF-α on arterial thrombogenesis.
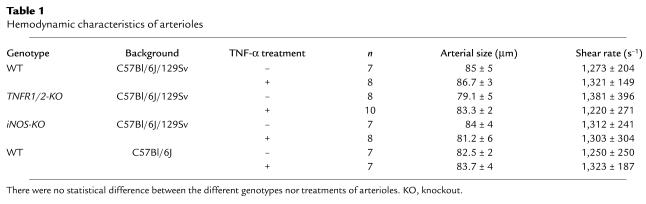
The effect of TNF-α on thrombus formation was assessed in an in vivo model of ferric chloride–induced arterial injury. Fluorescently labeled platelets were injected into mice in the presence or the absence of TNF-α (1 ng/ml). Arterioles were chosen to display similar hemodynamic characteristics (Table 1). Injury was induced with ferric chloride within 30 minutes after the injection of the cytokine. In both control and TNF-α–treated mice, platelet interactions with the injured vessel started rapidly after ferric chloride application, and the number of single adherent platelets found in the monitored areas 2–3 minutes after injury was not significantly different between the two groups (Figures 1 and 2a). The time required for the first appearance of a 10-μm diameter thrombus in TNF-α–treated mice, however, was approximately three times longer than in control animals (Figure 2a). Furthermore, thrombi in treated mice were unstable and failed to resist shear stress while growing to a larger size, constantly dissociating into single platelets or very small emboli. In the control mice thrombi grew to occlusive size in approximately 14 minutes, whereas vessel occlusion was significantly delayed in TNF-α–treated animals (Figures 1 and 2a) (14 ± 3 minutes in control mice versus 33 ± 6 minutes in treated mice). The same experiment was performed in WT mice of a different background (C57Bl/6J versus C57Bl/6J/129Sv) where the thrombotic response to injury was also reduced by TNF-α treatment, indicating that the antithrombotic effect of TNF-α is not dependent on the background of the mice used in our studies. The injection of 1 ng/ml of TNF-α did not induce a decrease in platelet count. Our data show that, under high-shear conditions found in arterioles (approximately 1,300 s–1), TNF-α does not influence the initial transient platelet adhesion to the injured vessel wall but significantly inhibits thrombus growth and stability.
Table 1.
Hemodynamic characteristics of arterioles
Figure 1.
Effect of TNF-α on thrombus formation in vivo. Thrombus formation in response to vascular injury was visualized in arterioles of control (upper) or TNF-α–treated mice (lower). The different lengths of time after ferric chloride application are indicated. No significant difference in initial platelet adhesion to the injured vessel was observed between the two groups (3 minutes). The appearance of thrombi was delayed by TNF-α infusion (8 minutes), however. In addition, thrombi in treated mice were unstable and often did not grow to occlusive size, leaving, after 14 minutes, a still patent vessel, whereas the control arterioles occluded. n = 12–15 mice.
Figure 2.
Quantitative analysis of arterial thrombogenesis in WT, TNF-R1/2–/–, L-NMMA–treated, and iNOS–/– mice. Two minutes after the injury, the number of platelet-vessel wall interactions in each group of mice was comparable between the TNF-α–treated and untreated animals (a–d). In contrast to the antithrombotic effect measured in WT mice (a), TNF-α did not significantly affect thrombus formation and vessel occlusion in TNF-R1/2–/– (b), L –NMMA–treated (c), and iNOS–/– mice (d). Compared with WT mice, iNOS–/– mice exhibited a significant increase in platelet deposition (P < 0.02) and a slightly shorter time in thrombus formation (P < 0.05) and in vessel occlusion (P < 0.03). n = 7–10 mice per group. *P < 0.05; **P < 0.005 versus untreated WT.
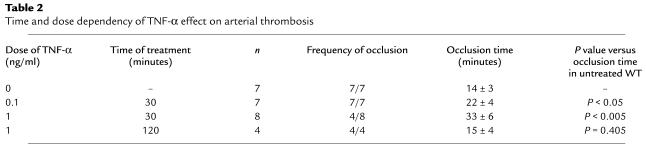
We subsequently investigated whether the antithrombotic effect of TNF-α was sustained over a longer period of treatment with 1 ng/ml of TNF-α. We did not observe any delay or defect in thrombus formation and occlusion time in animals treated with TNF-α 2 hours before injury, suggesting that the TNF-α effect is transient and reversible (Table 2).
Table 2.
Time and dose dependency of TNF-α effect on arterial thrombosis
To further characterize the inhibitory action of TNF-α, a lower dose of the cytokine was tested in the thrombosis model. At a dose of 0.1 ng/ml, such as is observed in nonlethal sepsis (18), TNF-α exerted an effect of a lesser magnitude on the thrombotic response of WT mice (Table 2). The time required for appearance of an arterial thrombus was slightly longer compared with control mice, but this difference was not significant (data not shown). These thrombi, however, grew with a significant delay of 8 minutes to occlusive size (Table 2).
Bleeding time in TNF-α−treated mice.
After amputation of a 3-mm portion of the tail, both untreated mice and mice treated with 1 ng/ml TNF-α were able to control their blood loss without cauterization. The bleeding time, however, was prolonged four times by the 30-minute treatment with TNF-α (254 ± 142 seconds versus 58 ± 11 seconds in the control group). Thus, the antithrombotic effect was also detected in this injury model (Figure 3a).
Figure 3.
Tail bleeding time and plasma clotting time in TNF-α–treated mice. Mice 12–16 weeks old were infused with PBS or TNF-α. (a) The bleeding time was measured after amputation of a 3-mm portion of the tail. The mice injected with TNF-α (filled circles) exhibited a fourfold prolonged bleeding time compared with control mice (open circles) (P < 0.003). (b) Plasma clotting time was measured in an aggregometer after adding CaCl2 solution. The clotting time was significantly shorter in mice treated with 1 ng/ml of TNF-α (black bar) than in control mice (white bar). P < 0.001, n = 8 mice per group.
TNF-α–generated procoagulant activity.
To evaluate whether the infusion of TNF-α in our model is associated with a procoagulant state, we measured the plasma-clotting time. Exposure to TNF-α reduced the clotting time by 44 seconds (Figure 3b; P < 0.001), indicating that the presence of high levels of circulating TNF-α indeed increased the coagulability of plasma. Because cell-derived microparticles can contribute to procoagulant activity (19–21) by allowing assembly of clotting factors, we examined the generation of microparticles. The plasma from TNF-α–treated mice showed a 1.6-fold increase in the total number of microparticles over those in control mice (489 ± 55 versus 306 ± 37 microparticles per microliter of plasma; n = 8, P < 0.007). Thus, we observed a prolonged bleeding time despite a procoagulant state of the TNF-α–treated mice (Figure 3).
Platelet P-selectin expression, fibrinogen binding, aggregation, and cGMP levels.
To elucidate the antithrombotic effect of TNF-α observed in vivo, we looked for a possible effect of TNF-α on platelet function in vitro. TNF-α (or PBS) was added to washed platelets or to whole blood and incubated for 30 minutes at 37°C. The treatment time of 30 minutes was chosen to parallel the incubation time in the mice used in the arterial thrombosis model. Platelet preparations were then stimulated with 0.1 U/ml thrombin or 2 μg/ml CRP and analyzed by flow cytometry for P-selectin expression and fibrinogen binding to the platelet surface. Surprisingly, we did not observe any difference in fibrinogen binding and P-selectin expression between PBS-and TNF-α–treated whole blood samples (Figure 4, a and c) or PRP samples (not shown). To test whether the inhibition observed in thrombus formation might be dependent on a dynamic environment, we also tested those platelet preparations at 1,000 rpm in an aggregometer (Figure 4e). No defect was noticed in the ADP-induced platelet aggregation in response to TNF-α treatment. Taken together, these data show that TNF-α does not affect platelet function when added to isolated platelets or whole blood, suggesting that cells other than blood cells were the mediators for the observed effect of TNF-α in vivo.
Figure 4.
Effect of in vivo and in vitro treatments with TNF-α on platelet function. PBS (white bars) or TNF-α (black bars) were administered for 30 minutes either to whole blood samples (a, c, and e) or by intravenous injection into mice (b, d, and f). PRP was prepared, and platelets were tested for aggregation in response to 2 μM ADP (e and f). To study platelet activation by flow cytometry, platelets were washed, activated for 5 minutes with thrombin (0.1 U/ml) or CRP (2 μg/ml), and incubated for 10 minutes at 37°C with FITC-conjugated Ab’s against human fibrinogen (a and b) or a FITC-conjugated mAb against P-selectin (c and d). (g and h) Shown are representative results of TNF-α systemic administration on fibrinogen binding and platelet aggregation. n = 6 mice per group. *P < 0.05; **P < 0.005. MFI, mean fluorescence intensity.
To test this hypothesis, mice were injected intravenously with 1 ng/ml of TNF-α or PBS 30 minutes before blood was drawn, and platelets were prepared as described above. Under these circumstances, both platelet fibrinogen binding and P-selectin expression were significantly reduced by TNF-α treatment in comparison with control samples (Figure 4, b, d, and g). In addition, platelets isolated from TNF-α–treated mice showed a 35% decrease in maximum aggregation compared with the PBS-injected animals (Figure 4, f and h). Shorter treatment of the mice (5 and 15 minutes) with the cytokine failed to impair the platelet function (not shown). Inhibition of platelet adhesion by agents such as NO seems to depend on the generation of cGMP (22), therefore we also examined the cGMP levels in platelets isolated from control and TNF-α−treated mice. Indeed a 30-minute exposure to circulating TNF-α induced an increase in the intraplatelet cGMP levels compared with control (2.1 ± 0.13 versus 1.54 ± 0.22 pmol/5 × 108 platelets; n = 5, P < 0.05), consistent with our platelet studies showing a partial decrease in platelet function.
Effect of TNF-α on arterial thrombogenesis in TNF-R1– and TNF-R2–deficient mice.
TNF-α mediates its effects through binding to its cognate receptors, TNF-R1 and TNF-R2 (12). To test whether TNF-Rs mediate the antithrombotic activity of TNF-α in vivo, we studied thrombus formation in TNF-R1 and TNF-R2 double-deficient mice. As shown in Figure 2b, TNF-α had no effect on thrombus formation and vessel occlusion in TNF-R1/2–/– mice. In addition, in the absence of TNF-α, the process of thrombus formation was not significantly different between the TNF-R1/2–/– mice (Figure 2b, white bar) and the corresponding WTs (Figure 2a, white bar), thus indicating that TNF-α receptors are not involved in thrombosis in the absence of inflammation. Furthermore, platelets isolated from TNF-R1/2–/– mice infused with TNF-α aggregated normally (not shown).
Arterial thrombosis in l-NMMA–treated and iNOS–/– mice.
Since we did not find evidence that TNF-α is acting directly on platelets, we investigated whether a mediator could be induced by TNF-α to downregulate platelet function. NO appeared to be a good candidate since it is a well-known inhibitor of platelet function (22). To address this hypothesis, we studied thrombus formation in mice injected with L-NMMA, an analogue of L-arginine that inhibits NO production by all isoforms of NOS. L-NMMA treatment itself did not significantly modify arterial thrombosis, but it completely abrogated the antithrombotic effect exerted by TNF-α treatment (Figure 2c). This indicates that the antithrombotic effect of TNF-α was mediated by NO. An isoform of NOS that is present in a variety of cell types and that is inducible by cytokines is iNOS; therefore, we examined thrombus formation in mice deficient in iNOS. Similar to the L-NMMA–treated mice, iNOS–/– mice grew stable thrombi leading to occlusion and were not affected by the TNF-α treatment (Figure 2d), suggesting that NO generated by TNF-α was produced by iNOS present in the vascular tissues. In addition, compared with WT mice, iNOS–/– mice exhibited a significant increase in the number of platelet interactions with the vessel wall (Figure 2d). Moreover, both thrombus formation and occlusion time occurred faster in those mice, suggesting that in WT mice NO might be released from the injured area and attenuate platelet recruitment to the growing thrombus.
Discussion
TNF-α exerts a strong antithrombotic activity in vivo.
In this study, intravital microscopy allowed us to demonstrate that treatment with TNF-α results in a marked antithrombotic effect in a mouse arterial injury model (Figures 1 and 2a). Consistent with this finding, the bleeding time of mice treated with TNF-α was significantly increased (Figure 3a). The dose of TNF-α used in our model (1 ng/ml of estimated blood volume) relates to plasma levels that can be reached in patients with septic conditions or related infectious disorders. In these patients, the presence of TNF-α has been correlated with a systemic activation of coagulation (23), which we observed as well in the treated mice (Figure 3b). Given the ability of TNF-α to promote coagulation (5), its inhibitory effect on thrombus formation in vivo was unexpected. Supporting the hypothesis that TNF-α is procoagulant but not prothrombotic, however, studies carried out in the baboon indicate that TNF-α is not able to elicit thrombosis unless the natural anticoagulant pathway is blocked (6, 24). Our results further indicate that the antithrombotic effect of TNF-α is fast and reversible, vanishing within 2 hours after infusion into mice (Table 2). In addition, our data point to a dose-dependent effect of the cytokine since vessel occlusion successfully occurred in all the animals treated with 100 pg/ml with a significant delay (Table 2).
The TNF-Rs mediating the antithrombotic activity of TNF-α are not located on blood cells.
Several steps were undertaken to elucidate the mechanism supporting the defect in thrombus formation. First, we checked that infusion of 1 ng/ml of TNF-α into mice did not induce a severe drop in the number of circulating platelets. In contrast to the administration of LPS (25) or significantly higher concentrations of TNF-α into mice (26), the platelet count did not drop in our study. In addition, TNF-α treatment appeared unlikely to affect the vascular injury because early platelet adhesions occurred efficiently. Several lines of evidence further indicated than TNF-α acts by downregulating platelet function. First, as observed in our in vivo injury model, platelets seemed to rapidly dissociate from the early growing thrombi decreasing the efficiency of their growth (Figure 1). Second, platelets isolated from TNF-α–treated mice exhibited, upon activation, a marked decrease in fibrinogen binding in comparison to controls, indicating that αIIbβ3 was less activated. Third, stimulated platelets exhibited lower P-selectin expression due to a lesser degranulation (Figure 4, b and d). Not surprisingly, ADP-induced aggregation of the in vivo–treated platelets was also diminished (Figure 4f). In contrast, in vitro treatment of either isolated platelets or whole blood samples with TNF-α had no effect on platelet activation by thrombin, CRP, or ADP (Figure 4, a, c, and e), demonstrating that TNF-α does not act directly on platelets but through another cell type other than blood cells. As for the receptors involved in mediating the antithrombotic effect of TNF-α, we considered two likely candidates, TNF-R1 and TNF-R2, and, based on the similarity with CD40L, the platelet integrin αIIbβ3 (9). Since TNF-R1/2–/– mice infused with TNF-α exhibited no significant defect in thrombus formation (Figure 2b), we concluded that the inhibitory effect of TNF-α was mediated through the TNF-Rs.
NO is the platelet inhibitor produced by TNF-α.
The next step in our study was to define the mechanism through which TNF-α mediates its inhibitory effect on platelets. NO is a well-known inhibitor of platelet function (22). By activation of guanylyl cyclase, inhibition of the PI3K pathway, and suppression of intracellular calcium flux, NO suppresses both P-selectin expression and the conformational change in glycoprotein αIIbβ3 required for fibrinogen binding (22). Indeed, platelets obtained from TNF-α–treated mice had elevated cGMP levels, indicating that guanylyl cyclase was activated in these cells. Since NO is induced in response to TNF-α through stimulation of iNOS present in a variety of cell types (27), we first investigated the possible involvement of NO as a mediator of TNF-α activity on thrombosis by testing the effect of TNF-α in mice treated with L-NMMA. Interestingly, L-NMMA treatment completely prevented the antithrombotic effect of TNF-α, suggesting that NO plays a critical role in inflammation by regulating platelet thrombus formation (Figure 2c). A second line of evidence came from studies with mice lacking iNOS (Figure 2d). Despite the infusion of TNF-α, these mice were still capable of forming occlusive thrombi. Our data further indicate that NO modulates thrombus formation in injured vessels even in the absence of TNF-α treatment because iNOS–/– mice exhibited an increased number of platelet-vessel wall interactions and a slightly shorter time required for both thrombus appearance and vessel occlusion (Figure 2d). Thus, it appears that a deficiency of vascular NO formation promotes arterial thrombosis. Small amounts of NO might be released at the site of injury after the ferric chloride application, thereby locally attenuating platelet recruitment. This possibility is further supported by observations that platelet deposition on injured vessels is enhanced if NO is cleared by hemoglobin (28) or NOS is inhibited (29), while platelet deposition is prevented when NO production is enhanced by treatment with L-arginine, the substrate for iNOS (29).
Different cell types found in the surroundings of the vessel, such as endothelial cells, VSMCs, and leukocytes present in the subendothelial space, can express iNOS (27, 30). Under inflammatory conditions such as sepsis, LPS, or TNF-α treatment, however, the strongest expression of iNOS is in VSMCs, whereas its production is inhibited in endothelial cells (27, 30). It is likely that, in our arterial model, the VSMCs expressing TNF-Rs are the cells that respond to TNF-α and generate NO. In support of this hypothesis, iNOS mRNA can be detected in the VSMCs as early as 20 minutes after LPS challenge (31), timing that is compatible with the response to TNF-α administration in our model. Since LPS-induced iNOS mRNA expression occurs rapidly, and given that many effects of LPS are mediated through the production of TNF-α, we may expect TNF-α to have a similar or even a faster effect. In addition, different studies further suggest that low levels of iNOS mRNA could be present constitutively in VSMCs (32, 33). This could explain the rapid release of NO in response to the vascular injury.
Biological significance of TNF-α antithrombotic effect.
Given the central role of TNF-α both as a regulator of the normal immune response and as an inducer of the chronic damage in organs produced in a variety of diseases, modulation of this cytokine became a therapeutic approach of choice. In chronic inflammatory diseases such as rheumatoid arthritis, Crohn disease, or multiple sclerosis (34–36), where anti–TNF-α therapies are used, thrombotic side effects were not reported. It is possible that in such chronic situations the “beneficial” antithrombotic effect of TNF-α is lost. In contrast, the effect of TNF-α on the vasculature could be present in antitumor therapies where TNF-α is administered locally to cancer patients through isolated limb perfusion. Surprisingly, no deleterious effects on the vasculature have been observed in these patients (37). This could be explained by the acute antithrombotic effect of TNF-α observed in our study and by the recent observation that NO inhibits secretion of Weibel-Palade bodies, thus diminishing platelet adhesion to the endothelium (38, 39). In light of our results, it should be considered that treatments targeting TNF-α could influence the prothrombotic potential of a patient.
Although the biological significance of the transient antithrombotic effect of TNF-α remains to be elucidated, it is possible to interpret its inhibitory effect on platelet function in the context of innate immune response. TNF-α is the first proinflammatory cytokine released at the site of infection or injury and is a potent inducer of the immune defense mechanisms, including the recruitment and activation of leukocytes (40). Under these circumstances, a platelet monolayer would help in recruitment (41), while a thrombus would restrict blood flow and could physically prevent a full access of migrating leukocytes to the infected site. The widely held perception that TNF-α has prothrombotic effects mainly stems from the fact that TNF-α is considered to be the major endogenous mediator involved in the pathogenesis of sepsis and related disorders such as disseminated intravascular coagulation, in which a hypercoagulable state exists (42). In addition, our observation of prolonged bleeding time in the TNF-α–treated mice indicates that enhanced coagulation induced by TNF-α cannot overcome the platelet defect induced by NO.
In conclusion, our study points to a new inflammatory function of TNF-α in transiently inhibiting thrombosis, thus allowing the immune response to be optimally executed.
Acknowledgments
We thank Crystal Piffath for technical assistance, Dorathy F. Vargas for mouse husbandry, and Lesley Cowan for help preparing the manuscript. This work was supported in part by NIH National Heart, Lung and Blood Institute grants R01-HL-53756 and R37-HL-41002 (to D.D. Wagner) and by a fellowship from Emmy-Noether Programms der Deutschen Forschungsgemeinschaft (to W. Bergmeier).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: TNF receptor-1 (TNF-R1); TNF receptor (TNF-R); CD40 ligand (CD40L); NG-monomethyl-l-arginine (l-NMMA); amino acids Lys-Gly-Asp (KGD); collagen-related peptide (CRP); platelet-rich plasma (PRP); platelet-poor plasma (PPP).
References
- 1.Vallet B, Wiel E. Endothelial cell dysfunction and coagulation. Crit. Care Med. 2001;29(Suppl.):S36–S41. doi: 10.1097/00003246-200107001-00015. [DOI] [PubMed] [Google Scholar]
- 2.Ruberg FL, Leopold JA, Loscalzo J. Atherothrombosis: plaque instability and thrombogenesis. Prog. Cardiovasc. Dis. 2002;44:381–394. doi: 10.1053/pcad.2002.123469. [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3:27–34. doi: 10.1016/s1470-2045(01)00619-2. [DOI] [PubMed] [Google Scholar]
- 4.Korte W. Veno-occlusive disease of the liver after bone marrow transplantation: is hypercoagulability really part of the problem? Blood Coagul. Fibrinolysis. 1997;8:367–381. doi: 10.1097/00001721-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Esmon CT. Possible involvement of cytokines in diffuse intravascular coagulation and thrombosis. Baillieres Best Pract. Res. Clin. Haematol. 1999;12:343–359. doi: 10.1053/beha.1999.0029. [DOI] [PubMed] [Google Scholar]
- 6.Esmon CT. Does inflammation contribute to thrombotic events? Haemostasis. 2000;30 (Suppl.):34–40. doi: 10.1159/000054161. [DOI] [PubMed] [Google Scholar]
- 7.Philippe C, et al. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am. J. Pathol. 1993;143:1713–1723. [PMC free article] [PubMed] [Google Scholar]
- 8.Tacchini-Cottier F, Vesin C, Redard M, Buurman W, Piguet PF. Role of TNFR1 and TNFR2 in TNF-induced platelet consumption in mice. J. Immunol. 1998;160:6182–6186. [PubMed] [Google Scholar]
- 9.Andre P, et al. CD40L stabilizes arterial thrombi by a beta3 integrin-dependent mechanism. Nat. Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 10.Bergmeier W, Rackebrandt K, Schroder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 2000;95:886–893. [PubMed] [Google Scholar]
- 11.Morton LF, Hargreaves PG, Farndale RW, Young RD, Barnes MJ. Integrin alpha 2 beta 1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for alpha 2 beta 1-independent platelet reactivity. Biochem. J. 1995;306:337–344. doi: 10.1042/bj3060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peschon JJ, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 13.Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denis C, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni H, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J. Clin. Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramaniam M, et al. Defects in hemostasis in P-selectin-deficient mice. Blood. 1996;87:1238–1242. [PubMed] [Google Scholar]
- 17.Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13835–13840. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohler KM, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J. Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 19.Combes V, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satta N, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J. Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 21.Giesen PL, et al. Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 23.Billiau A, Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur. J. Clin. Invest. 1991;21:559–573. doi: 10.1111/j.1365-2362.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 24.Wakefield TW, et al. Deep venous thrombosis in the baboon: an experimental model. J. Vasc. Surg. 1991;14:588–598. doi: 10.1067/mva.1991.32030. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, et al. LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system. Blood. 2002;100:3233–3239. doi: 10.1182/blood-2002-01-0252. [DOI] [PubMed] [Google Scholar]
- 26.Piguet PF, Vesin C, Da Kan C. Activation of platelet caspases by TNF and its consequences for kinetics. Cytokine. 2002;18:222–230. doi: 10.1006/cyto.2002.0889. [DOI] [PubMed] [Google Scholar]
- 27.Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc. Res. 1999;43:509–520. doi: 10.1016/s0008-6363(99)00161-3. [DOI] [PubMed] [Google Scholar]
- 28.Olsen SB, et al. Enhancement of platelet deposition by cross-linked hemoglobin in a rat carotid endarterectomy model. Circulation. 1996;93:327–332. doi: 10.1161/01.cir.93.2.327. [DOI] [PubMed] [Google Scholar]
- 29.Yan ZQ, Yokota T, Zhang W, Hansson GK. Expression of inducible nitric oxide synthase inhibits platelet adhesion and restores blood flow in the injured artery. Circ. Res. 1996;79:38–44. doi: 10.1161/01.res.79.1.38. [DOI] [PubMed] [Google Scholar]
- 30.MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem. Biophys. Res. Commun. 1993;196:1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- 31.Liu SF, Barnes PJ, Evans TW. Time course and cellular localization of lipopolysaccharide-induced inducible nitric oxide synthase messenger RNA expression in the rat in vivo. Crit. Care Med. 1997;25:512–518. doi: 10.1097/00003246-199703000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Gosgnach W, Messika-Zeitoun D, Gonzalez W, Philipe M, Michel JB. Shear stress induces iNOS expression in cultured smooth muscle cells: role of oxidative stress. Am. J. Physiol. Cell Physiol. 2000;279:C1880–C1888. doi: 10.1152/ajpcell.2000.279.6.C1880. [DOI] [PubMed] [Google Scholar]
- 33.Chyu KY, et al. Decreased neointimal thickening after arterial wall injury in inducible nitric oxide synthase knockout mice. Circ. Res. 1999;85:1192–1198. doi: 10.1161/01.res.85.12.1192. [DOI] [PubMed] [Google Scholar]
- 34.Raza A. Anti-TNF therapies in rheumatoid arthritis, Crohn’s disease, sepsis, and myelodysplastic syndromes. Microsc. Res. Tech. 2000;50:229–235. doi: 10.1002/1097-0029(20000801)50:3<229::AID-JEMT6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885–1888. doi: 10.1212/wnl.57.10.1885. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm. Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Lejeune FJ, Ruegg C, Lienard D. Clinical applications of TNF-alpha in cancer. Curr. Opin. Immunol. 1998;10:573–580. doi: 10.1016/s0952-7915(98)80226-4. [DOI] [PubMed] [Google Scholar]
- 38.Matsushita K, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide sensitive factor. Cell. 2003;115:1–20. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andre P, et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood. 2000;96:3322–3328. [PubMed] [Google Scholar]
- 40.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 41.Yeo EL, Sheppard JA, Feuerstein IA. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model) Blood. 1994;83:2498–2507. [PubMed] [Google Scholar]
- 42.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]