Abstract
Spermatogenesis in the adult male depends upon the action of follicle stimulating hormone (FSH) and androgen. Ablation of either hormone has deleterious effects on Sertoli cell function and the progression of germ cells through spermatogenesis. In this study we have generated mice lacking both FSH receptors (FSHRKO) and androgen receptors on the Sertoli cell (SCARKO) to examine how FSH and androgen combine to regulate Sertoli cell function and spermatogenesis. Sertoli cell number in FSHRKO.SCARKO mice was reduced by about 50% but was not significantly different to FSHRKO mice. In contrast, total germ cell number in FSHRKO.SCARKO mice was reduced to 2% of control mice (and 20% of SCARKO mice) due to a failure to progress beyond early meiosis. Measurement of Sertoli cell-specific transcript levels showed that about a third were independent of hormonal action on the Sertoli cell while others were predominantly androgen-dependent or showed redundant control by FSH and androgen. Results show that FSH and androgen act through redundant, additive and synergystic regulation of spermatogenesis and Sertoli cell activity. In addition, the Sertoli cell retains a significant capacity for activity which is independent of direct hormonal regulation.
Introduction
In the adult animal Sertoli cells act primarily to promote and maintain germ cell development. This is achieved by generation of a unique microenvironment within the seminiferous tubules through the formation of a Sertoli cell barrier and regulation of solute movement and secretion into the tubules (1;2). In addition, they provide cytoarchitectural support and stimulation to the developing germ cells as they undergo proliferation and differentiation. Previous studies have shown that spermatogenesis is regulated by follicle-stimulating hormone (FSH) and testosterone and that these hormones act through specific receptors on the Sertoli cell (reviewed in (3)). The role that each of these hormones plays in the regulation of Sertoli cell function and spermatogenesis has become clearer through study of mice lacking specific hormones or hormone receptors. In animals lacking FSH (FSHβKO) or the FSH receptor (FSHRKO) there is a reduction in Sertoli cell number and germ cell number but the animals remain fertile (4-7). In contrast, absence of the androgen receptor (AR), either ubiquitously through a mutation in the receptor (Tfm) or specifically in the Sertoli cells (SCARKO) will cause arrest of spermatogenesis in early meiosis (8-11). Thus, FSH appears to act to induce proliferation of the Sertoli cells and germ cells and to “optimise” the progress of spermatogenesis while testosterone action on the Sertoli cell is critical for progression through meiosis.
While it is clear that both FSH and androgen are essential for normal spermatogenesis, what remains unknown is the nature and importance of interaction between the hormones. For example, the degree of overlap, or redundancy, is uncertain and also, therefore, the extent to which ablation of one hormone or its receptor may be compensated by the presence of the other hormone. Similarly, the degree to which Sertoli cell function and germ cell development is independent of direct hormonal stimulation remains unclear. To answer these questions directly we have generated mice lacking both FSHR and AR on the Sertoli cell (FSHRKO.SCARKO) and have determined the subsequent effects on Sertoli cell function and spermatogenesis.
Materials & Methods
Animals and treatments
All mice were bred and all procedures carried out under UK Home Office Licence and with the approval of a local ethical review committee. Mice with a specific Sertoli cell knockout of the AR have been previously generated by crossing male mice expressing AMH.Cre (12) with female mice carrying an AR with a floxed exon 2 (ARfl ) (9). In order to produce male mice lacking both FSHR and AR within the Sertoli cell, mice carrying the AMH.Cre transgene (C57-BL6/SJL) and mice carrying the ARfl allele (Swiss-Webster/129) were crossed with FSHRKO mice (C57-BL6/129) (6) and interbred. The groups used for comparison with the double knockout FSHRKO.SCARKO mice were a) hemizygous FSHRKO/+ males (FSHRH) expressing AMH.Cre or ARfl which were considered as control animals (the two groups were initially analysed separately but no significant difference between the AMH.Cre and ARfl groups was seen and the data were combined) b) FSHRKO.Cre and FSHRKO.ARfl which were considered as FSHRKO animals and c) FSHRH.SCARKO animals which were considered as SCARKO animals for this study. PCR genotyping was carried out as previously described (9;13).
Mice were killed at eight weeks and testes snap frozen in liquid nitrogen or fixed overnight. Fixation was either in Bouin's for subsequent morphometric analysis or 4% paraformaldehyde/1% gluteraldehyde in phosphate buffer (0.1M, pH 7.2) for preparation of semi-thin sections.
Hormone measurements
Blood was collected by cardiac puncture under anaesthesia and the serum separated and stored at −20°C until assayed. Serum and pituitary levels of FSH and LH were measured commercially using imunofluorimetric assays (Delfia, Wallac OY, Turku, Finland) as previously described (14;15). Serum levels of testosterone were measured by radioimmunoassay following ether extraction (16).
Measurement of specific mRNA transcript levels
To quantify the content of specific mRNA species in testes from each group, a real-time PCR approach was used after reverse transcription (RT) of the isolated RNA. To allow specific mRNA levels to be expressed per Sertoli cell and to control for the efficiency of RNA extraction, RNA degradation, and the RT step, an external standard was used (7;17;18). The external standard was luciferase mRNA (Promega UK, Southampton, UK), and 5 ng was added to each testis at the start of the RNA extraction procedure. Testis RNA was extracted using TRIzol (Life Technologies, Paisley, UK) and the RNA was reverse transcribed using random hexamers and Moloney murine leukemia virus reverse transcriptase (Superscript II, Life Technologies, Paisley, UK) as described previously (19). For real-time PCR the SYBR green method was used in a 96-well plate format using a Stratagene MX3000 cycler. Reactions contained 5 μl 2 × SYBR mastermix (Stratagene, Amsterdam, Netherlands), primer (100 nM) and template in a total volume of 10 μl. At the end of the amplification phase a melting curve analysis was carried out on the products formed. All primers were selected using Primer Express 2.0 (Applied Biosystems, Warrington, UK) with parameters previously described (20) and were designed so that the amplicon would cross at least one intron. The primers used have been described previously (21;22). To correct for Sertoli cell number data from the real-time PCR studies was divided by the Sertoli cell number of each group as measured below.
Histology and stereology
To prepare semi-thin (1μm) sections testes were embedded in araldite and sections stained with toluidine blue. For stereological analysis, testes were embedded in Technovit 7100 resin, cut into sections (20μm), and stained with Harris' hematoxylin. The total testis volume was estimated using the Cavalieri principle (23). The optical disector technique (24) was used to count the number of Sertoli cells and germ cells in each testis. Each cell type was identified by previously described criteria (25;26). The numerical density of each cell type was estimated using an Olympus BX50 microscope fitted with a motorized stage (Prior Scientific Instruments, Cambridge, UK) and Stereologer software (Systems Planning Analysis, Alexandria, VA, USA).
Statistical analysis
Data were analysed using two factor analysis of variance (anova) with each gene knockout as one of the factors. Where the interaction between the factors was significant this means that the effect of the double knockout was not simply an additive effect of each gene knockout alone. To show whether differences between individual groups were significant t-tests were employed using the pooled variance from the anova. Data were log transformed where appropriate to avoid heterogeneity of variance.
Results
Phenotype
Adult FSHRKO.SCARKO mice were normally masculinised although testes from the double knockouts were significantly smaller than FSHRKO, SCARKO or control mice (Table 1). Seminal vesicle weights were also slightly smaller in FSHRKO.SCARKO mice (Table 1). Seminiferous tubule diameter decreased across the groups in the order control>FSHRKO>SCARKO>FSHRKO.SCARKO with an associated increase in the relative abundance of interstitial tissue (Fig 1B). All stages of spermatogenesis were present in FSHRKO mice while, in SCARKO mice, there was apparent loss of pachytene spermatocytes with a marked reduction in the number of secondary spematocytes and few round spermatids present (Fig 1B). In FSHRKO.SCARKO mice germ cells entered meiosis but development stopped at early pachytene for most cells with no secondary spermatocytes or round spermatids apparent (Fig 1B).
Table 1.
Organ weights in 8 week-old mice
| Control | FSHRKO | SCARKO | FSHRKO. SCARKO |
|
|---|---|---|---|---|
| Testis weight | 87.3 ± 1.9a | 36.9 ± 1.3b | 20.6 ± 0.4c | 8.1 ± 0.2d |
| SV weight | 196 ± 5a | 183 ± 6ab | 190 ± 5a | 172 ± 5b |
| N | 53 | 36 | 65 | 38 |
Within each row, groups with different letter superscripts are significantly different. There was a significant interaction between the effects of FSHRKO and SCARKO on testis weight.
Fig 1.
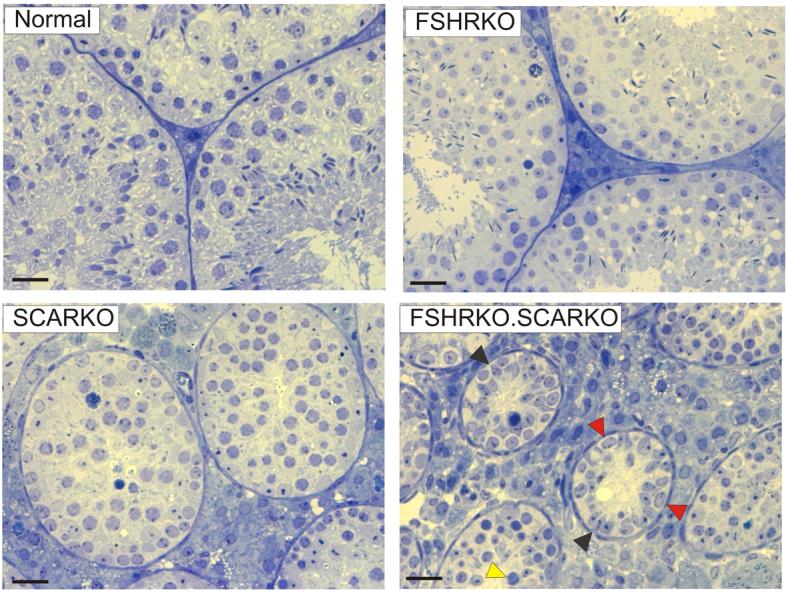
Gross and morphological appearance of testes from 8 week old mice. Semithin sections from testes of 8 week old Normal, FSHRKO, SCARKO and FSHRKO.SCARKO mice. The FSHRKO mice contained all stages of spermatogenesis although germ cell number was reduced. In SCARKO mice spermatogenesis progressed through meiosis but there was progressive loss of pachytene spermatocytes and few secondary spermatocytes or round spermatids were observed. In FSHRKO.SCARKO mice the tubules were of a smaller diameter with large numbers of Sertoli cells (black arrowheads) and smaller numbers of spermatogonia (red arrowheads). Spermatogonia entered meiosis but development stopped at early pachytene in most cells (yellow arrowhead). The bar represents 20μm.
Morphometric analysis showed that Sertoli cell number in 8-week old FSHRKO.SCARKO mice was similar to FSHRKO mice and significantly less than control or SCARKO mice (Fig 2). Sertoli cells in SCARKO and FSHRKO.SCARKO mice contained large lipid droplets (not shown) that were not seen in other groups. The total germ cell number was reduced in FSHRKO and SCARKO mice compared to control and there was a further marked reduction in FSHRKO.SCARKO mice (Fig 2). The germ cell/Sertoli cell ratio was reduced, though not markedly, in FSHRKO mice compared to control. In contrast, in SCARKO mice the germ cell/Sertoli cell ratio was reduced to 10% of control and in the FSHRKO.SCARKO it was further reduced to 2% of control (Fig 2). Analysis of germ cell types in each group showed that spermatogonial numbers were reduced by about 60% in the FSHRKO and FSHRKO.SCARKO mice (Fig 3). Spermatocyte number was also reduced by about 50% in FSHRKO and SCARKO mice but in the combined FSHRKO.SCARKO mouse there was very limited successful progression into meiosis with spermatocyte numbers reduced to 4% of control (Fig 3). The number of round spermatids was reduced to about 40% in the FSHRKO mouse with very few (<0.5% of control) post-meiotic cells present in the SCARKO mouse and none in the FSHRKO.SCARKO mouse.
Fig 2.
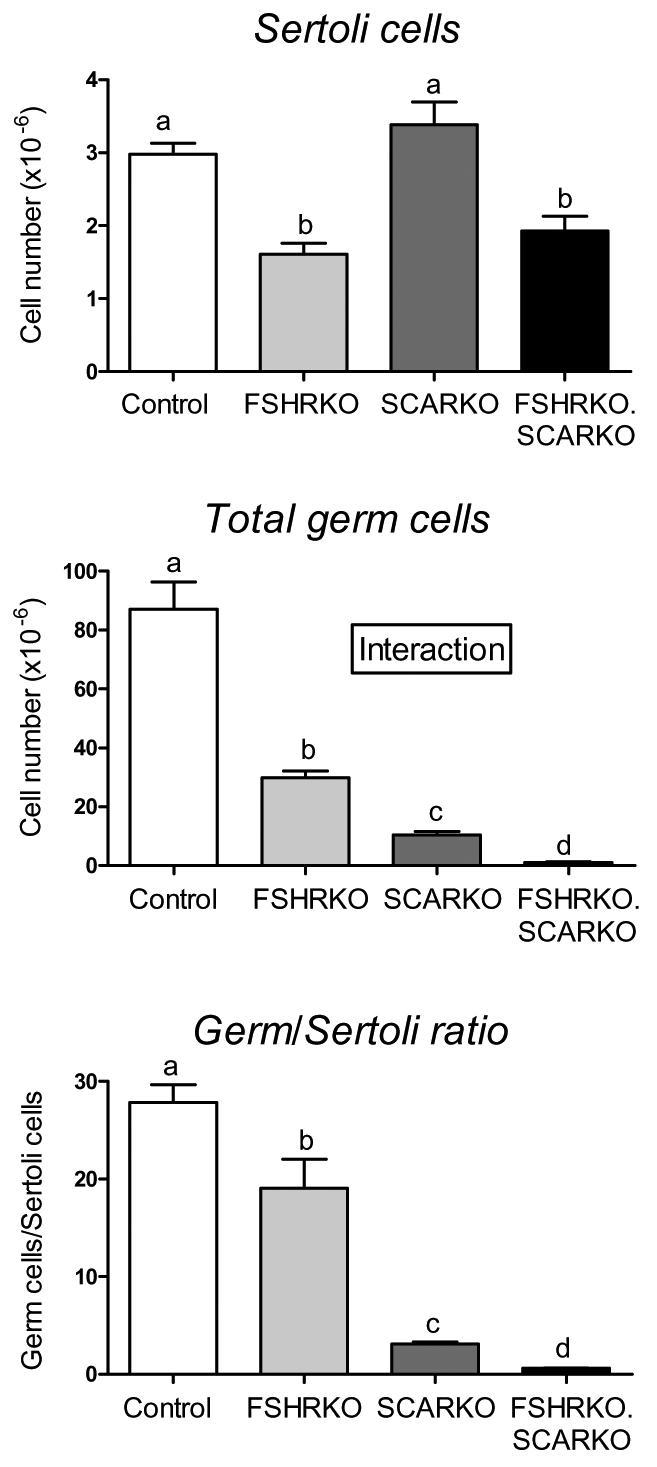
Morphometric analysis Sertoli and germ cell numbers in 8 week old testes from control, FSHRKO, SCARKO and FSHRKO.SCARKO mice. Cell number were measured using the optical disector method. Results show the mean ± sem of 4 animals per group. Groups with different letter superscripts are significantly different. Where there was a significant interaction between the effects of the two gene knockouts this is indicated on the figure.
Fig 3.
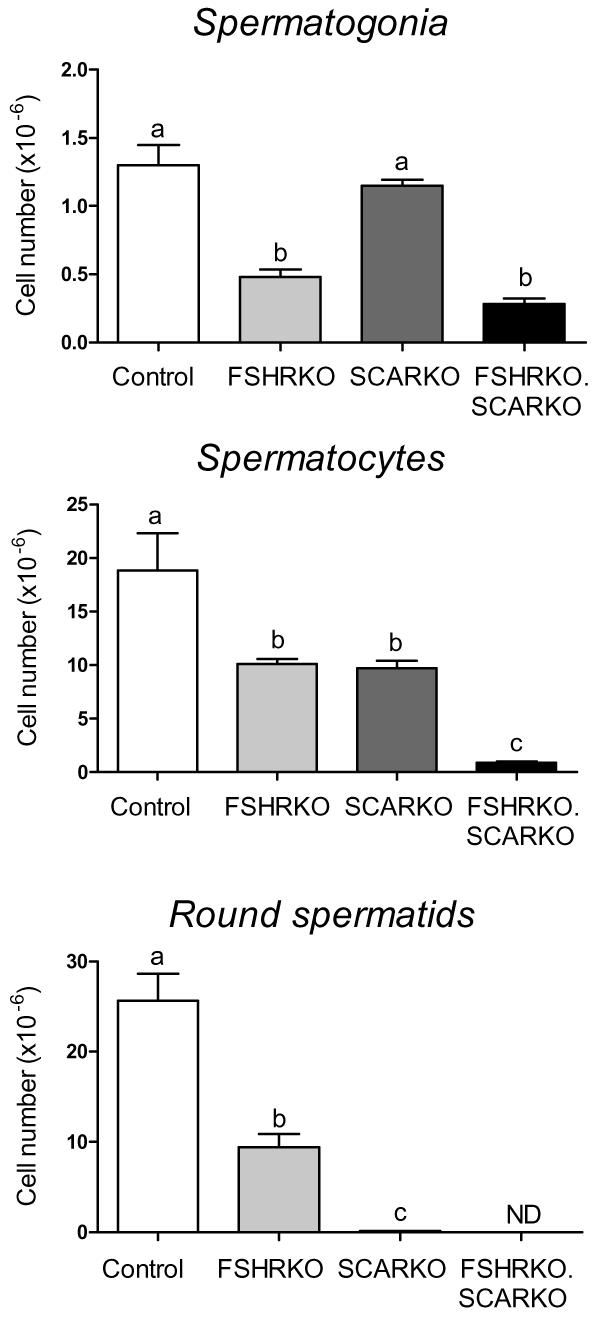
Morphometric analysis of germ cell types in 8 week old testes from control, FSHRKO, SCARKO and FSHRKO.SCARKO mice. Cell number were measured using the optical disector method. Results show the mean ± sem of 4 animals per group. Groups with different letter superscripts are significantly different.
Hormone profile
Serum levels of LH were significantly elevated in FSHRKO mice but were not significantly affected in SCARKO mice. In FSHRKO.SCARKO mice LH levels were significantly greater than all other groups (Table 2). There were no significant differences in serum FSH or testosterone levels between groups.
Table 2.
Serum hormone levels in 8 week old animals
| Group | Testosterone (pmol/ml) |
LH* (ng/ml) |
FSH (ng/ml) |
|---|---|---|---|
| Control | 4.2 ± 1.7 | 0.064 ± 0.012a | 35.7 ± 11.1 |
| FSHRKO | 5.1 ± 3.5 | 0.286 ± 0.085b | 11.9 ± 3.8 |
| SCARKO | 8.5 ± 2.4 | 0.221 ± 0.065ab | 32.6 ± 12.3 |
| FSHRKO.SCARKO | 3.0 ± 1.4 | 0.586 ± 0.086c | 17.1 ± 5.46 |
Groups with different letter superscripts are significantly different. There was no interaction between the effects of the two gene knockouts.
Results show mean ± sem of 5 to 8 animals in each group
Sertoli cell mRNA levels
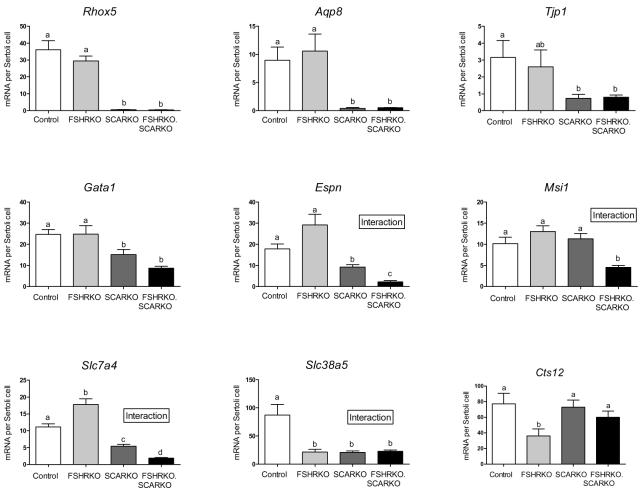
To examine Sertoli cell function in the 4 groups, real-time PCR was used to measure the levels of 14 mRNA transcripts which are specifically or predominantly expressed in the Sertoli cells. Transcript levels were measured relative to an external control and data normalised for Sertoli cell number (7) (Table 3, Fig 4). Five of the transcripts tested (Sox9, Itgb1,Wt1, Trf, and Dhh) showed no significant difference in abundance per Sertoli cell between the 4 groups although between-animal variation was relatively high for some transcripts (Table 3). Of the remaining 9 transcripts, 4 showed a significant alteration in levels related only to the presence of the AR (Rhox5, Aqp8, Tjp1 and Gata1), one transcript responded to the loss of either FSHR or AR (Slc38a5), one responded only to the loss of FSHR (Cts12) and 3 transcripts (Espn, Msi1 and Slc7a4) showed a significantly more marked response to the loss of both receptors than to either FSHR or AR alone (Fig 4).
Table 3.
mRNA transcript levels in 8 week old mice – transcripts unaffected in FSHRKO, SCARKO or FSHRKO.SCARKO mice
| Group |
||||
|---|---|---|---|---|
| Gene | Control | FSHRKO | SCARKO | FSHRKO. SCARKO |
| Sox9 | 9.1 ± 2.0 | 9.4 ± 4.4 | 7 .4 ± 0.8 | 12.0 ± 1.4 |
| Wt1 | 16.1 ± 3.7 | 20.4 ± 3.7 | 19.1 ± 3.4 | 15.7 ± 2.0 |
| Itgb1 | 8.1 ± 2.7 | 6.6 ± 1.0 | 5.3 ± 0.5 | 5.2 ± 1.3 |
| Trf | 4.9 ± 1.7 | 5.8 ± 1.4 | 4.1 ± 1.1 | 5.4 ± 1.0 |
| Dhh | 2.8 ± 0.3 | 3.7 ± 0.9 | 2.3 ± 1.2 | 2.5 ±0.4 |
Data shows mRNA transcript levels per Sertoli cell
Fig 4.
Levels of Sertoli cell-specific mRNA transcripts in testes from 8 week old control, FSHRKO, SCARKO and FSHRKO.SCARKO mice. Transcripts levels were measured relative to an external standard by real-time PCR and corrected for Sertoli cell number as described in Methods. Results show the mean ± sem of 5 or 6 animals per group. Transcripts in A) showed no significant difference in expression per Sertoli cell between groups while transcripts in B) showed significant variation. In B) the groups with different letter superscripts are significantly different. Where there was a significant interaction between the effects of the two gene knockouts this is indicated on the figure.
Discussion
Generation of the FSHRKO.SCARKO mouse provides us with a base from which to examine how FSH and androgen act and interact through the Sertoli cell to regulate testicular function. In addition, it allows us to determine what aspects of Sertoli cell function are independent of direct hormonal input. The phenotype shows there is additive, synergistic and redundant regulation of spermatogenesis and the Sertoli cell by FSH and androgen but that regulation of a significant number of Sertoli cell transcripts (and, by implication, Sertoli cell function) is independent of direct hormonal control.
Interactions between the effects of FSH and androgens on testicular function have been studied previously using models such as the hypophysectomised rat or the hypogonadal (hpg) mouse which lacks gonadotrophin-releasing hormone (GnRH) with consequent loss of circulating gonadotrophins (27). The advantage of the FSHRKO.SCARKO mouse, in contrast to these models, is the specificity and/or totality of the knockout effects. All current data suggests that the FSHR is confined to the Sertoli cells in the male (28;29) while SCARKO mice have been generated to lack ARs only in the Sertoli cells (9). Thus, the targeted ablations affect only the Sertoli cells in this double knockout and while there may be knock-on effects on gonadotrophin levels or androgen production these are not complicating factors since the Sertoli cells are un-responsive to hormonal stimulation.
Effectively, the FSHRKO.SCARKO mouse provides a baseline control from which the direct effects of FSH and androgen on Sertoli cell function can be assessed. Thus, in the SCARKO mouse the Sertoli cells are stimulated by FSH and not androgen, in the FSHRKO mouse they are stimulated by androgen and not FSH while in the normal mouse they are exposed to both hormones. From this perspective it can be seen that the action of FSH is to increase Sertoli cell number, total germ cell number and the number of germ cells associated with each Sertoli cell. This effect is achieved by increasing the number of spermatogonia and by enhancing the entry of these cells into meiosis. In contrast, androgens have no direct effect on Sertoli cell number but cause a marked increase in total germ cell number and, thus, the number of germ cells per Sertoli cell. The principal effect appears to be through increased entry into meiosis and, crucially, by enabling completion of meiosis. Together, the hormones have an additive effect on entry into meiosis but act synergistically to stimulate completion of meiosis and entry into spermiogenesis. In addition, it is clear from the double knockout that germ cells can initiate meiosis without direct hormonal stimulation through the Sertoli cell, supporting the conclusions of early in vitro studies (30). The general description of hormone action on spermatogenesis is also consistent with earlier hormone replacement studies which have shown, for example, that FSH and androgen will increase or restore spermatocyte number (31-33) while androgens will increase the number of post-meiotic germ cells in the hpg mouse (31;34).
Development of a normal sized cohort of Sertoli cells is of importance for male fertility since each Sertoli cell can only support a finite number of germ cells. Previous studies have shown that both FSH and androgen can act to regulate the final Sertoli cell number in the adult animal (7;35) although initial studies on the SCARKO mouse showed that androgen-dependent regulation of Sertoli cell number does not appear to be through direct effects of androgen on the Sertoli cell (7;9;36). In FSHRKO.SCARKO mice the number of Sertoli cells was identical to the FSHRKO mouse showing that only FSH is of importance in terms of direct effects on Sertoli cell proliferation with no synergistic or additive effect of androgen apparent. The nuclei of the Sertoli cells in the FSHRKO.SCARKO mice appeared to take up more of a pseudostratified appearance due, probably, to the reduced diameter of the tubules. This marked reduction in tubular diameter is unlikely to be due, entirely, to loss of germ cells in the FSHRKO.SCARKO as tubules in the germ cell-free W/Wv mouse are less markedly affected and the Sertoli cells maintain a simple epithelium (37;38 and unpublished observation) This suggests that FSH and androgen regulate the diameter of the seminiferous tubules and maintain the normal morphology of the epithelium.
The significant increase in LH levels in FSHRKO mice has been reported previously (39) and attributed to reduced testosterone production by the testis. In FSHRKO.SCARKO mice the increase in LH was more marked and may be indicative of further dysfunction in the Leydig cells. Both SCARKO and FSHRKO mice have reduced Leydig cell number (39;40) and it is possible that the effects are additive in the FSHRKO.SCARKO mouse although this will require further study. Circulating testosterone levels were unchanged in both FSHRKO and FSHRKO.SCARKO but there was high variability between animals and a more reliable indicator of androgen levels may be the reduction in weight of the seminal vesicles in FSHRKO.SCARKO mice. Reduced Leydig cell number or function in the SCARKO, FSHRKO and FSHRKO.SCARKO mice is further evidence for a hormone-dependent Sertoli cell factor which regulates Leydig cell function (41;42).
Despite our enhanced understanding of the role played by FSH and androgens in the regulation of spermatogenesis the cellular mechanisms involved are still in some doubt. It is clear that FSH can regulate levels of a large number of different mRNA transcripts (43) and the overall effect of FSH may be to increase general Sertoli cell activity and, thereby, enhance germ cell progression and survival. In contrast, only a relatively small number of Sertoli cell genes have been shown unequivocally by array studies to be androgen-dependent in a number of different mouse models (22;44-47). Somewhat confusingly, there is little overlap of identified androgen-dependent genes between these studies. It is likely that this is due to differences in the age of the animals used and to differences in the endocrine environment or previous hormonal exposure of the animals. Nevertheless, there is some accumulating evidence to suggest that androgens are required for functional generation of the Sertoli cell barrier and for development of the specialised tubular environment required for germ cell development (22;48). Thus, in contrast to FSH, androgen action may be mediated through a relatively small number of changes in Sertoli cell gene expression. To examine the effect of ablating both FSHR and AR on Sertoli cell activity we measured the abundance of 14 mRNA species known to be expressed specifically or predominantly within the Sertoli cell population in the adult testis (21;22;49). The 14 transcripts divided into 3 groups, those showing no significant response to ablation of either or both receptors, those sensitive to loss of the AR and those sensitive to loss of both receptors. The relatively large number of transcripts (∼35%) which were unaffected by loss of hormone responsiveness indicates that a significant proportion of Sertoli cell activity may be independent of hormone action. In addition, 20% of transcripts (Espn, Msi1 and Slc7a4) showed a degree of redundancy between the effects of FSH and androgen. Overall, therefore, extrapolating from this data it appears likely that a significant number of Sertoli cell genes are either hormone independent or can be stimulated by either hormone which may act to ensure an adequate baseline of cell activity irrespective of fluctuations in hormone levels. When considering changes in Sertoli cell activity following alterations in hormone or hormone receptor levels a possible confounding factor is the effect of germ cell loss on Sertoli cell mRNA transcript levels (50;51). All of the transcripts reported in this study, however, have been shown to be unaffected by germ cell ablation (21).
The transcripts shown in Fig 5 were selected for study largely because they are expressed specifically or predominantly in the Sertoli cells and illustrate different aspects of hormonal regulation of the cell. Nevertheless, the altered expression of some of these transcripts, particularly in the SCARKO and FSHRKO.SCARKO mice, offers some further clues to the specific mechanism of hormonal action on the Sertoli cell. ESPN, for example, is an actin-bundling protein (52) and an integral part of the ectoplasmic specialisations which are specific to the Sertoli cell and contribute to the Sertoli cell (blood testis) barrier (53). Similarly, TJP1 is integral to the structure of tight junctions which are a major component of the Sertoli cell barrier (54). The reduction in Espn and Tjp1 levels in the SCARKO and FSHRKO.SCARKO mouse would, therefore, be likely to contribute to the overall disruption of the Sertoli cell barrier seen in the absence of the AR (48).
The marked loss of Aqp8 transcripts in the SCARKO was surprising since previous array studies have not identified Aqp8 as androgen dependent (22;44;47). This discrepancy may have come about, however, because earlier array studies used immature testes (10d and 20d) and expression of Aqp8 only begins around day 16 in the rat testis (55). The aquaporins are a family of water-transporting proteins which act to allow osmotically-driven movement of water across cell membranes or epithelial layers. Interestingly, in mice lacking AQP8 there is a significant enlargement of the testis (56) although whether this is due to changes in fluid accumulation or cell numbers is unknown. Loss of Aqp8 in the SCARKO mouse and the pattern of expression of the solute carriers (Slc7a4 and Slc38a5) in the different groups is further evidence that both FSH and androgens act directly or indirectly to regulate the internal environment of the seminiferous tubules and, thereby, facilitate germ cell development (22).
This study shows that spermatogenesis is dependent upon the action of FSH and androgen on the Sertoli cell with only the initial onset of meiosis apparently independent of direct hormonal regulation. The two hormones can both act to maintain the meiotic germ cell population but there is an absolute need for androgen to complete meiosis. Spermiogenesis does not appear to require FSH since the ratio of round spermatids to mature sperm in the FSHRKO is similar to control (not shown) though the models are not informative on the role of androgen in this process. Since other cell types in the testis express the AR it is also possible that androgens may have additional indirect effects on spermatogenesis and this will require further study of FSHRKO.ARKO mice.
Acknowledgements
This study was supported by the Wellcome Trust. We thank Prof DG de Rooij for assistance with identification of germ cell morphology.
Gene names
- Aqp8
Aquaporin 8
- Cst12
Cystatin 12
- Dhh
Desert hedgehog
- Espn
Espin
- Gata1
GATA binding protein 1
- Itgb1
β1 Integrin
- Msi 1
Musashi homolog 1
- Rhox5
Reproductive homeobox 5
- Slc7a4
Solute carrier family 7a4
- Slc38a5
Solute carrier family 38a5
- Sox9
SRY-box containing gene 9
- Tjp1
Tight junction protein 1
- Trf
Transferrin
- Wt1
Wilms tumor homolog
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
Reference List
- 1.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 2.Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 5.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 7.Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 8.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 9.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis. 2002;33:114–118. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- 13.Hirst RC, Abel MH, Wilkins V, Simpson C, Knight PG, Zhang FP, Huhtaniemi I, Kumar TR, Charlton HM. Influence of mutations affecting gonadotropin production or responsiveness on expression of inhibin subunit mRNA and protein in the mouse ovary. Reproduction. 2004;128:43–52. doi: 10.1530/rep.1.00176. [DOI] [PubMed] [Google Scholar]
- 14.Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- 15.van Casteren JI, Schoonen WG, Kloosterboer HJ. Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol Reprod. 2000;62:886–894. doi: 10.1095/biolreprod62.4.886. [DOI] [PubMed] [Google Scholar]
- 16.O'Shaughnessy PJ, Sheffield JW. Effect of testosterone on testicular steroidogenesis in the hypogonadal (hpg) mouse. J Steroid Biochem. 1990;35:729–734. doi: 10.1016/0022-4731(90)90315-j. [DOI] [PubMed] [Google Scholar]
- 17.Baker PJ, O'Shaughnessy PJ. Expression of prostaglandin D synthetase during development in the mouse testis. Reproduction. 2001;122:553–559. doi: 10.1530/rep.0.1220553. [DOI] [PubMed] [Google Scholar]
- 18.O'Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod. 2002;66:966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- 19.O'Shaughnessy PJ, Murphy L. Cytochrome P-450 17α-hydroxylase protein and mRNA in the testis of the testicular feminized (Tfm) mouse. J Mol Endocrinol. 1993;11:77–82. doi: 10.1677/jme.0.0110077. [DOI] [PubMed] [Google Scholar]
- 20.Czechowski T, Bari RP, Stitt M, Scheible WR, Udvardi MK. Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J. 2004;38:366–379. doi: 10.1111/j.1365-313X.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Shaughnessy PJ, Hu L, Baker PJ. Effect of germ cell depletion on levels of specific mRNA transcripts in mouse Sertoli cells and Leydig cells. Reproduction. 2008 doi: 10.1530/REP-08-0012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Shaughnessy PJ, Abel M, Charlton HM, Hu B, Johnston H, Baker PJ. Altered expression of genes involved in regulation of vitamin a metabolism, solute transportation, and cytoskeletal function in the androgen-insensitive tfm mouse testis. Endocrinology. 2007;148:2914–2924. doi: 10.1210/en.2006-1412. [DOI] [PubMed] [Google Scholar]
- 23.Mayhew TM. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- 24.Wreford NG. Theory and practice of stereological techniques applied to the estimation of cell number and nuclear volume of the testis. Microsc Res Tech. 1995;32:423–436. doi: 10.1002/jemt.1070320505. [DOI] [PubMed] [Google Scholar]
- 25.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. pp. 119–161. [Google Scholar]
- 26.Baker PJ, O'Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- 27.Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadtrophin releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- 28.Heckert LL, Griswold MD. The expression of the follicle-stimulating hormone receptor in spermatogenesis. Recent Prog Horm Res. 2002;57:129–148. doi: 10.1210/rp.57.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter AM, Lukyanenko YO, Lee VH, Hutson JC. FSH does not directly influence testicular macrophages. J Androl. 1998;19:420–427. [PubMed] [Google Scholar]
- 30.Hofmann M-CC, Dym M. Long-term culture of mammalian spermatogonia. In: Skinner MK, Griswold MD, editors. Sertoli cell biology. San Diego: Elsevier; 2005. pp. 449–470. [Google Scholar]
- 31.Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology. 2003;144:509–517. doi: 10.1210/en.2002-220710. [DOI] [PubMed] [Google Scholar]
- 32.Russell LD, Corbin TJ, Borg KE, De Franca LR, Grasso P, Bartke A. Recombinant human follicle-stimulating hormone is capable of exerting a biological effect in the adult hypophysectomized rat by reducing the numbers of degenerating germ cells. Endocrinology. 1993;133:2062–2070. doi: 10.1210/endo.133.5.8404654. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. The effects of recombinant follicle-stimulating hormone on the restoration of spermatogenesis in the gonadotropin-releasing hormone-immunized adult rat. Endocrinology. 1995;136:4035–4043. doi: 10.1210/endo.136.9.7649112. [DOI] [PubMed] [Google Scholar]
- 34.Singh J, Oneill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (Hpg) Mice. Endocrinology. 1995;136:5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- 35.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 36.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G. The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total (ARKO) or Sertoli cell-selective (SCARKO) ablation of the androgen receptor. Endocrinology. 2005;146:2674–2683. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 37.Safina F, Tanaka S, Inagaki M, Tsuboi K, Sugimoto Y, Ichikawa A. Expression of L-histidine decarboxylase in mouse male germ cells. J Biol Chem. 2002;277:14211–14215. doi: 10.1074/jbc.M200702200. [DOI] [PubMed] [Google Scholar]
- 38.Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod. 2000;63:1185–1191. doi: 10.1095/biolreprod63.4.1185. [DOI] [PubMed] [Google Scholar]
- 39.Baker PJ, Pakarinen P, Huhtaniemi IT, Abel MH, Charlton HM, Kumar TR, O'Shaughnessy PJ. Failure of Normal Leydig Cell Development in Follicle-Stimulating Hormone (FSH) Receptor-Deficient Mice, But Not FSHbeta-Deficient Mice: Role for Constitutive FSH Receptor Activity. Endocrinology. 2003;144:138–145. doi: 10.1210/en.2002-220637. [DOI] [PubMed] [Google Scholar]
- 40.De Gendt K, Atanassova N, Tan KA, De Franca LR, Parreira GG, McKinnell C, Sharpe RM, Saunders PT, Mason J, Hartung S, Ivell R, Denolet E, Verhoeven G. Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective (SCARKO) or total (ARKO) ablation of the androgen receptor. Endocrinology. 2005;146:4117–4126. doi: 10.1210/en.2005-0300. [DOI] [PubMed] [Google Scholar]
- 41.Vihko KK, Lapolt PS, Nishimori K, Hsueh AJ. Stimulatory effects of recombinant follicle-stimulating hormone on Leydig cell function and spermatogenesis in immature hypophysectomized rats. Endocrinology. 1991;129:1926–1932. doi: 10.1210/endo-129-4-1926. [DOI] [PubMed] [Google Scholar]
- 42.O'Shaughnessy PJ, Bennett MK, Scott IS, Charlton HM. Effects of FSH on Leydig cell morphology and function in the hypogonadal mouse. Journal of Endocrinology. 1992;135:517–525. doi: 10.1677/joe.0.1350517. [DOI] [PubMed] [Google Scholar]
- 43.Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD. Follicle-stimulating hormone induced changes in gene expression of murine testis. Mol Endocrinol. 2004;18:2805–2816. doi: 10.1210/me.2003-0203. [DOI] [PubMed] [Google Scholar]
- 44.Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders PT, Swinnen JV, Verhoeven G. The effect of a Sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol. 2006;20:321–334. doi: 10.1210/me.2005-0113. [DOI] [PubMed] [Google Scholar]
- 45.Eacker SM, Shima JE, Connolly CM, Sharma M, Holdcraft RW, Griswold MD, Braun RE. Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol. 2007;21:895–907. doi: 10.1210/me.2006-0113. [DOI] [PubMed] [Google Scholar]
- 46.Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD. Identification of testosterone-regulated genes in testes of hypogonadal mice using oligonucleotide microarray. Mol Endocrinol. 2004;18:422–433. doi: 10.1210/me.2003-0188. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod. 2005;72:1010–1019. doi: 10.1095/biolreprod.104.035915. [DOI] [PubMed] [Google Scholar]
- 48.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beardsley A, O'Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- 50.Jonsson CK, Zetterstrom RH, Holst M, Parvinen M, Soder O. Constitutive expression of interleukin-1alpha messenger ribonucleic acid in rat Sertoli cells is dependent upon interaction with germ cells. Endocrinology. 1999;140:3755–3761. doi: 10.1210/endo.140.8.6900. [DOI] [PubMed] [Google Scholar]
- 51.Maguire SM, Millar MR, Sharpe RM, Saunders PT. Stage-dependent expression of mRNA for cyclic protein 2 during spermatogenesis is modulated by elongate spermatids. Mol Cell Endocrinol. 1993;94:79–88. doi: 10.1016/0303-7207(93)90054-n. [DOI] [PubMed] [Google Scholar]
- 52.Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109(Pt 6):1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- 53.Sluka P, O'Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J Endocrinol. 2006;189:381–395. doi: 10.1677/joe.1.06634. [DOI] [PubMed] [Google Scholar]
- 54.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 55.Kageyama Y, Ishibashi K, Hayashi T, Xia G, Sasaki S, Kihara K. Expression of aquaporins 7 and 8 in the developing rat testis. Andrologia. 2001;33:165–169. doi: 10.1046/j.1439-0272.2001.00443.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang B, Song Y, Zhao D, Verkman AS. Phenotype analysis of aquaporin-8 null mice. Am J Physiol Cell Physiol. 2005;288:C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]