Abstract
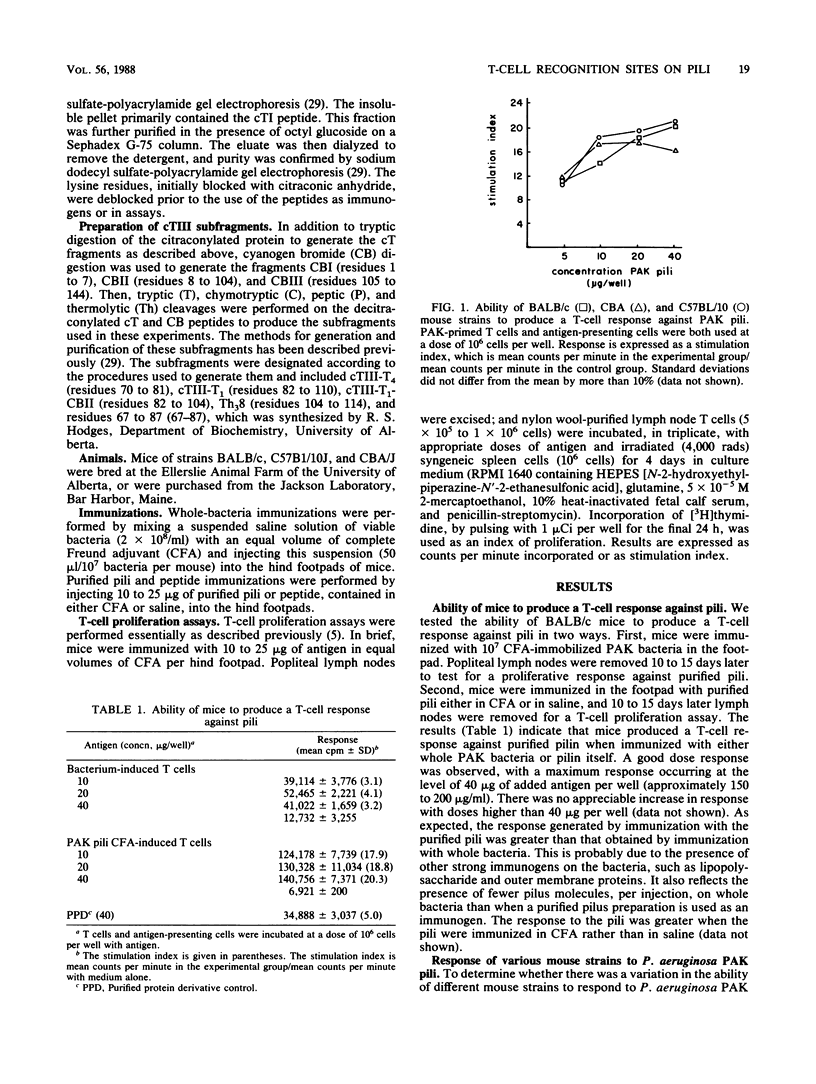
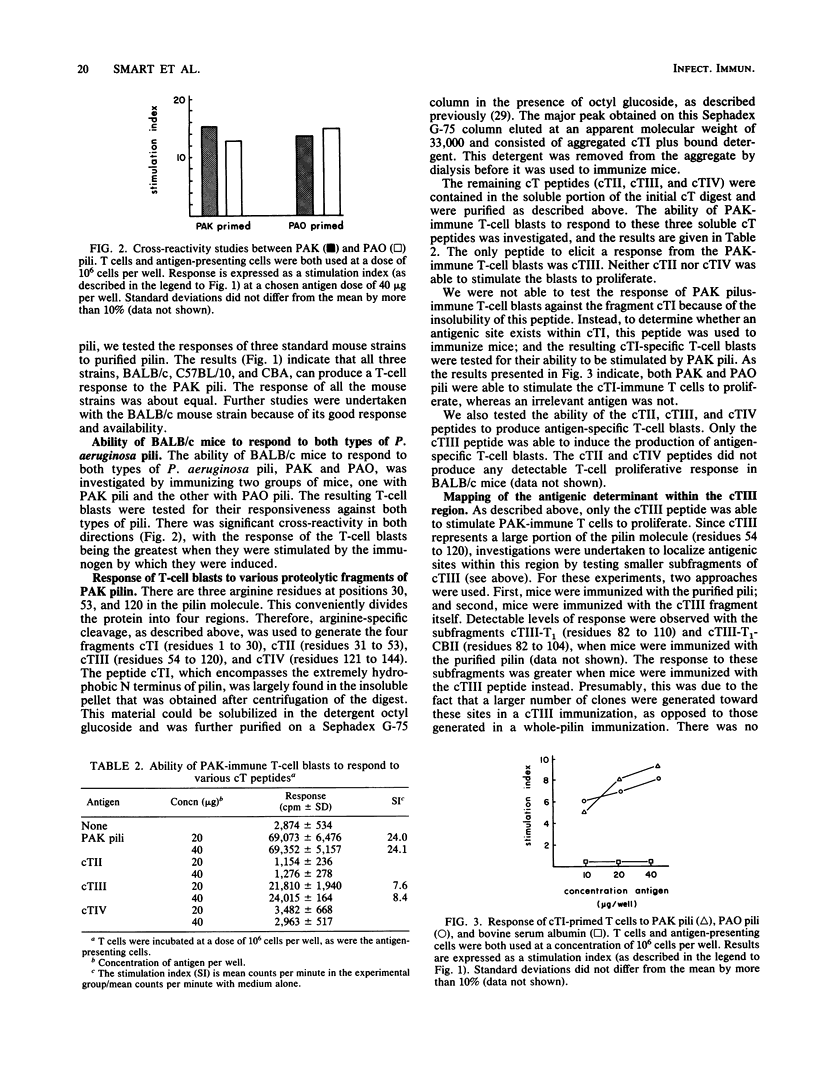
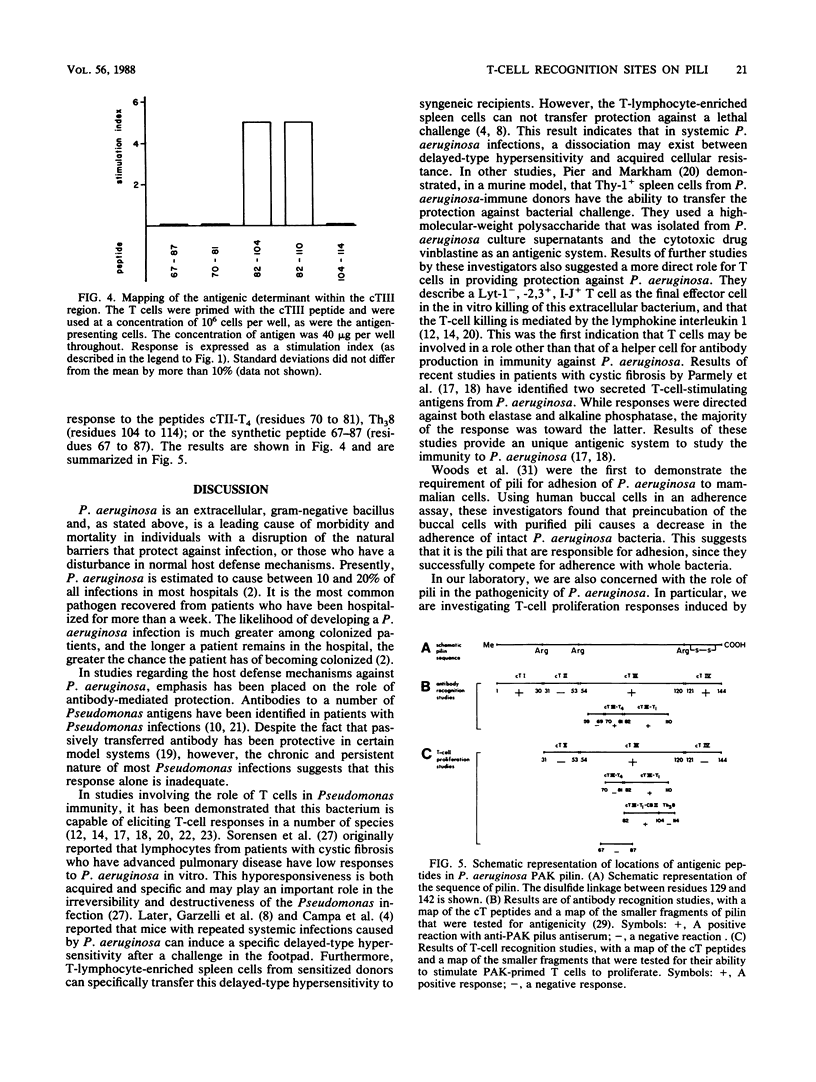
The polar pili of Pseudomonas aeruginosa consist of a subunit protein, pilin, which is a 144-residue polypeptide that contains a hydrophobic N-terminal region and eight hydrophilic regions distributed throughout the remainder of the molecule. T cells from mice immunized with pili or whole bacteria gave good pilus-specific T-cell proliferation responses. To delineate the T-cell antigenic regions of the pilin, T-cell blasts were generated from lymph nodes of pilus-primed BALB/c mice. These blasts were tested in vitro in T-cell proliferation assays for reactivity against the fragments of the pilin subunit prepared by enzymatic digestion. Citraconylation followed by trypsin digestion (cT) of the pilin subunit cleaved the protein into four fragments, cTI (residues 1 to 30), cTII (residues 31 to 53), cTIII (residues 54 to 120), and cTIV (residues 121 to 144). The ability to stimulate the T cells was found to reside in the cTI and cTIII regions, but not in the cTII or cTIV regions. A subfragment of cTIII, containing residues 82 to 104, was identified as the major T-cell recognition site within the cTIII region of the pilin molecule. A cross-reactivity was observed between pili from two strains of P. aeruginosa, namely, PAK and PAO, at the T-cell level. This cross-reactivity probably resulted from the sequence homology in the hydrophobic N-terminal region of these two molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk R. S., Hazlett L. D. Further studies on the genetic control of murine corneal response to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S936–S940. doi: 10.1093/clinids/5.supplement_5.s936. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Campa M., Toca L., Lombardi S., Garzelli C., Colizzi V., Falcone G. Cell-mediated immunity and delayed-type hypersensitivity in Pseudomonas aeruginosa-infected mice. Med Microbiol Immunol. 1982;170(3):191–199. doi: 10.1007/BF02298199. [DOI] [PubMed] [Google Scholar]
- Fotedar A., Boyer M., Smart W., Widtman J., Fraga E., Singh B. Fine specificity of antigen recognition by T cell hybridoma clones specific for poly-18: a synthetic polypeptide antigen of defined sequence and conformation. J Immunol. 1985 Nov;135(5):3028–3033. [PubMed] [Google Scholar]
- Froholm L. O., Sletten K. Purification and N-terminal sequence of a fimbrial protein from Moraxella nonliquefaciens. FEBS Lett. 1977 Jan 15;73(1):29–32. doi: 10.1016/0014-5793(77)80008-2. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977 Jul;131(1):259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzelli C., Colizzi V., Campa M., Falcone G. Mouse footpad infection by Pseudomonas aeruginosa: evidence for delayed hypersensitivity to specific bacterial antigen. Med Microbiol Immunol. 1980;168(2):111–118. doi: 10.1007/BF02121759. [DOI] [PubMed] [Google Scholar]
- Kilgannon P. D., Fraga E., Singh B. Fine-specificity analysis of antibodies directed to the C-terminal peptides of cytochrome c recognized by T-lymphocytes. Mol Immunol. 1986 Mar;23(3):311–318. doi: 10.1016/0161-5890(86)90058-1. [DOI] [PubMed] [Google Scholar]
- Klinger J. D., Straus D. C., Hilton C. B., Bass J. A. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: Demonstration by radioimmunoassay. J Infect Dis. 1978 Jul;138(1):49–48. doi: 10.1093/infdis/138.1.49. [DOI] [PubMed] [Google Scholar]
- Kurisaki J., Atassi H., Atassi M. Z. T cell recognition of ragweed allergen Ra3: localization of the full T cell recognition profile by synthetic overlapping peptides representing the entire protein chain. Eur J Immunol. 1986 Mar;16(3):236–240. doi: 10.1002/eji.1830160305. [DOI] [PubMed] [Google Scholar]
- Markham R. B., Goellner J., Pier G. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. I. Evidence that a lymphokine mediates killing. J Immunol. 1984 Aug;133(2):962–968. [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Goellner J. J., Mizel S. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. II. The role of macrophages and T cell subsets in T cell killing. J Immunol. 1985 Jun;134(6):4112–4117. [PubMed] [Google Scholar]
- Markham R. B., Pier G. B. Immunologic basis for mouse protection provided by high-molecular-weight polysaccharide from immunotype 1 Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S957–S962. doi: 10.1093/clinids/5.supplement_5.s957. [DOI] [PubMed] [Google Scholar]
- McKern N. M., O'Donnell I. J., Inglis A. S., Stewart D. J., Clark B. L. Amino acid sequence of pilin from Bacteroides nodosus (strain 198), the causative organism of ovine footrot. FEBS Lett. 1983 Nov 28;164(1):149–153. doi: 10.1016/0014-5793(83)80039-8. [DOI] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Frost L. S., Carpenter M., Armstrong G. D., Watts T. H. Biochemical studies on pili isolated from Pseudomonas aeruginosa strain PAO. Can J Microbiol. 1979 Oct;25(10):1175–1181. doi: 10.1139/m79-182. [DOI] [PubMed] [Google Scholar]
- Parmely M. J., Horvat R. T. Antigenic specificities of Pseudomonas aeruginosa alkaline protease and elastase defined by human T cell clones. J Immunol. 1986 Aug 1;137(3):988–994. [PubMed] [Google Scholar]
- Parmely M. J., Iglewski B. H., Horvat R. T. Identification of the principal T lymphocyte-stimulating antigens of Pseudomonas aeruginosa. J Exp Med. 1984 Nov 1;160(5):1338–1349. doi: 10.1084/jem.160.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B. Induction in mice of cell-mediated immunity to Pseudomonas aeruginosa by high molecular weight polysaccharide and vinblastine. J Immunol. 1982 May;128(5):2121–2125. [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwoll J. M., Gebel H. M., Rodey G. E., Markham R. B. In vitro response of human T cells to Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):670–674. doi: 10.1128/iai.40.2.670-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powderly W. G., Pier G. B., Markham R. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. III. The role of suppressor T cells in nonresponder mice. J Immunol. 1986 Jan;136(1):299–303. [PubMed] [Google Scholar]
- Sastry P. A., Finlay B. B., Pasloske B. L., Paranchych W., Pearlstone J. R., Smillie L. B. Comparative studies of the amino acid and nucleotide sequences of pilin derived from Pseudomonas aeruginosa PAK and PAO. J Bacteriol. 1985 Nov;164(2):571–577. doi: 10.1128/jb.164.2.571-577.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry P. A., Pearlstone J. R., Smillie L. B., Paranchych W. Studies on the primary structure and antigenic determinants of pilin isolated from Pseudomonas aeruginosa K. Can J Biochem Cell Biol. 1985 Apr;63(4):284–291. doi: 10.1139/o85-042. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen R. U., Stern R. C., Polmar S. H. Cellular immunity to bacteria: impairment of in vitro lymphocyte responses to Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1977 Dec;18(3):735–740. doi: 10.1128/iai.18.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Kay C. M., Paranchych W. Spectral properties of three quaternary arrangements of Pseudomonas pilin. Biochemistry. 1983 Jul 19;22(15):3640–3646. doi: 10.1021/bi00284a016. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Sastry P. A., Hodges R. S., Paranchych W. Mapping of the antigenic determinants of Pseudomonas aeruginosa PAK polar pili. Infect Immun. 1983 Oct;42(1):113–121. doi: 10.1128/iai.42.1.113-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. The structure and occurrence of pili (fimbriae) on Pseudomonas aeruginosa. J Gen Microbiol. 1971 Aug;67(2):135–143. doi: 10.1099/00221287-67-2-135. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


