Abstract
Dendritic cells (DCs) are antigen-presenting cells that play an essential role in mucosal tolerance. They regularly encounter beneficial intestinal bacteria, but the nature of these cellular contacts and the immune responses elicited by the bacteria are not entirely elucidated. Here, we examined the interactions of Lactobacillus acidophilus NCFM and its cell surface compounds with DCs. L. acidophilus NCFM attached to DCs and induced a concentration-dependent production of IL-10, and low IL-12p70. We further demonstrated that the bacterium binds to DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), a DC- specific receptor. To identify the DC-SIGN ligand present on the bacterium, we took advantage of a generated array of L. acidophilus NCFM mutants. A knockout mutant of L. acidophilus NCFM lacking the surface (S) layer A protein (SlpA) was significantly reduced in binding to DC-SIGN. This mutant incurred a chromosomal inversion leading to dominant expression of a second S layer protein, SlpB. In the SlpB-dominant strain, the nature of the interaction of this bacterium with DCs changed dramatically. Higher concentrations of proinflammatory cytokines such as IL-12p70, TNFα, and IL-1β were produced by DCs interacting with the SlpB-dominant strain compared with the parent NCFM strain. Unlike the SlpA-knockout mutant, T cells primed with L. acidophilus NCFM stimulated DCs produced more IL-4. The SlpA–DC-SIGN interaction was further confirmed as purified SlpA protein ligated directly to the DC-SIGN. In conclusion, the major S layer protein, SlpA, of L. acidophilus NCFM is the first probiotic bacterial DC-SIGN ligand identified that is functionally involved in the modulation of DCs and T cells functions.
Keywords: lactobacilli, DC-SIGN
Over a long evolutionary period, lactobacilli have been abundant colonizers of the human small intestinal mucosa coexisting in mutualistic relationships with the host. Some members of the group exert additional probiotic properties providing health benefits to the host via regulation of immune system functions. Of these, Lactobacillus acidophilus NCFM is one of the most widely recognized and commercially distributed probiotic cultures (1, 2). Although cell surface components of L. acidophilus NCFM and other lactobacilli resident in the human gastrointestinal (GI) tract could activate the functions of various antigen-presenting cells, the mechanisms of such immune modulations are largely unknown. The detailed characterization of L. acidophilus NCFM components that are effectors of the immune system is, therefore, critical for understanding host–microbial interplays and modes of action of commensal and probiotic bacteria in the intestine.
Dendritic cells (DCs) are professional antigen-presenting cells that regularly interact with intestinal bacteria, including lactobacilli at various mucosal sites. DCs play an essential role in bridging innate and adaptive immunity integrating various exogenous and indigenous stimuli and initiating the appropriate immune responses or tolerance (3, 4). Precursors of immature dendritic cells (iDCs) migrate through the bloodstream and home to various tissues including several compartments of the human gut where they can interact with protruding pathogenic and nonpathogenic bacteria. DCs then undergo phenotypic and functional changes, such as up-regulation of cell surface expression of costimulatory and adhesion molecules and production of inflammatory chemokines and cytokines. Along with antigen uptake and processing, these functional changes in the DCs initiate both humoral and adaptive immune responses. Depending on the microbial stimulus encountered, DCs can promote the differentiation of unprimed, naïve T cells toward Th1, Th2, unpolarized T cells, or T regulatory cell responses (4, 5).
A variety of pattern recognition receptors (PRRs) are expressed on iDCs recognizing characteristic molecular patterns within microbial carbohydrates, lipids, nucleic acids, and proteins of protruding pathogens or abundant commensal bacteria (6). Such receptors include the Toll-like receptors (TLRs) (7) and the C-type lectins (CLRs) (8). TLRs relay information from the interacting microbial compounds to DCs through intracellular signaling cascades, thereby eliciting appropriate cellular processes, such as DCs maturation and/or the induction of proinflammatory cytokines (IL-12, IFNγ) (7). In contrast, CLRs recognize carbohydrate structures on self- and nonself-antigens followed by their processing and presentation, without induction of DC maturation (9). Thus far, >15 CLRs have been identified on DCs and macrophages (10). DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN) is a CLR expressed mainly on DCs and recognizes mannose- and fucose-containing glycans that are present on endogenous and on microbial or viral surfaces. Because of these characteristics, DC-SIGN is implicated to play a role in the induction of various responses mediated by DCs. Several pathogens such as Helicobacter pilori, Mycobacterium tuberculosis, Streptococcus pneumoniae, Neisseria meningitides, and Schistosoma mansoni could interact with DC-SIGN and modulate responses (11–15).
Recently, it was found that Lactobacillus reuteri and Lactobacillus casei can also bind to DC-SIGN and lead to the induction of regulatory T cells (4). The detailed molecular mechanisms by which beneficial bacteria, including lactobacilli, interact with DC-SIGN to modulate immune responses and promote mucosal homeostasis are not completely understood. In this work, we investigated the molecular receptor/ligand interactions between the DCs and L. acidophilus NCFM and their contributions to the subsequent DC-driven Th1/Th2 differentiation.
Results
Cytokine Production by L. acidophilus NCFM-Treated DCs.
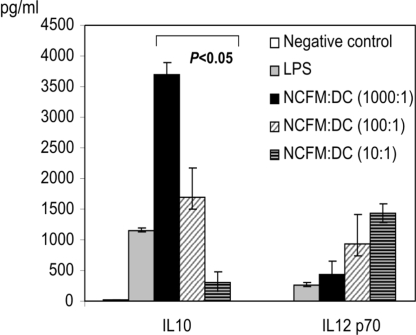
Previous studies have shown that DCs undergo maturation after interaction with lactobacilli, as measured by expression of cell surface markers and cytokines (5, 16, 17). To determine the levels of L. acidophilus NCFM attachment, bacteria were labeled with FITC and incubated at varying ratios, ranging from 1,000 to 10 cfu of L. acidophilus NCFM per 1 iDC, for 45min at 37 °C. Concentration-dependent bacterial binding was observed when the amount of the fluorescence detected by flow cytometry within the population of iDCs, was taken as a measure of the L. acidophilus NCFM adherence at the examined ratios [supporting information (SI) Fig. S1]. Furthermore, the effect of different concentrations of L. acidophilus NCFM on the expression of antiinflammatory (IL-10) and proinflammatory (IL-12p70) cytokines was assessed. The level of expression of these 2 cytokines was strongly influenced by the concentrations of L. acidophilus NCFM during the maturation of iDCs (Fig. 1) DCs incubated with the bacterium at ratio 1,000:1 (bacteria:iDCs) produced significantly higher IL-10 compared with a ratio of 10:1. In contrast, IL-12p70 was up-regulated at a lower concentration of the bacterium (10:1).
Fig. 1.
L. acidophilus NCFM induces concentration-dependent iDC cytokine responses. iDCs were incubated with L. acidophilus NCFM, LPS (10 ng/mL), or no supplement for 2 days at 37 °C. DCs supernatants were harvested after 48 h and analyzed for IL-10 and IL-12p70. Experiments were repeated 3 times, and the values are the average ± SD.
Next, the expression of HLA-DR and costimulatory molecules by L. acidophilus NCFM maturated DCs was examined. The bacteria modulated the iDC phenotype by up-regulating HLA-DR and activated the expression of the costimulatory molecules CD80 and CD86 at all examined ratios (1,000:1, 100:1 and 10:1; data not shown).
L. acidophilus NCFM Ligate DC-SIGN on Monocyte-Derived DCs via SlpA.
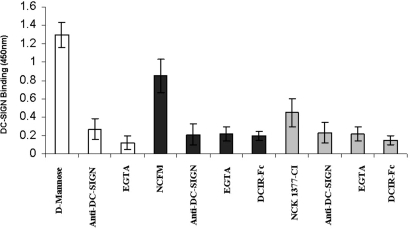
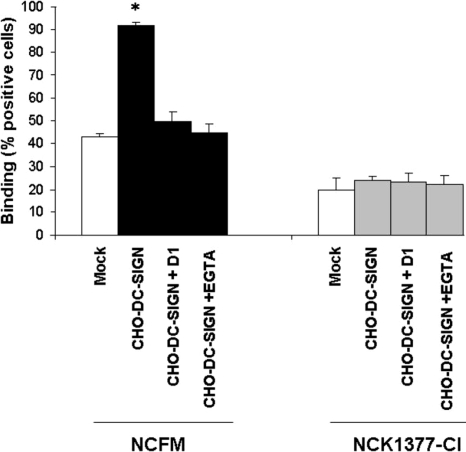
The role of particular PRRs in the bacterial–DC interactions was further evaluated. Specific C-type lectin receptors are highly expressed by DCs and bind carbohydrates on pathogens and commensals in a calcium-dependent manner. Ligation of L. acidophilus NCFM to iDCs was significantly reduced by the Ca2+ chelator EGTA, suggesting a role for the C-type lectins PRRs (Fig. S2). Earlier studies indicated that DC-SIGN is a major C-type lectin receptor on iDCs recognizing L. reuteri and L. casei (4). When the binding of L. acidophilus NCFM to this receptor was examined by using ELISA, a strong ligation of the bacteria to the DC-SIGN-Fc was observed (Fig. 2). Blocking studies revealed that this bacterium specifically interacted with DC-SIGN because neutralizing anti-DC-SIGN Ab or EGTA blocked the binding completely. Furthermore, no binding to L. acidophilus NCFM was obtained after using a DCs immunoreceptor fused to Fc (DCIR-Fc) as a control for the Fc binding in the ELISA (Fig. 2). To investigate whether cellular DC-SIGN binds L. acidophilus NCFM, we used CHO cells expressing DC-SIGN. Binding of L. acidophilus NCFM was significantly higher to CHO cell line transfected with DC-SIGN compared with mock (CHO cells alone) (Fig. 3). After the addition of DC-SIGN-specific antibody and EGTA, the L. acidophilus NCFM ligation to CHO cell line expressing DC-SIGN was reduced to the levels of attachment to mock. Thus, DC-SIGN functions as a cellular receptor for L. acidophilus NCFM.
Fig. 2.
Differential binding of DC-SIGN-Fc to L. acidophilus NCFM (SlpA+) and NCK1377-CI (SlpB+). By using the ELISA system, the DC-SIGN-Fc-binding specificity was determined in the presence of EGTA and blocking antibody to DC-SIGN (AZN-D1) and DCIR-Fc. L. acidophilus NCFM indicates L. acidophilus NCK1377-CI. All results are representative for 3 independent experiments (average ± SD); *, P < 0.05.
Fig. 3.
Cellular DC-SIGN is a receptor for L. acidophilus NCFM. CHO cells transfected with DC-SIGN and mock (CHO cells alone) (5 × 104 cells) were treated with 5 × 106 FITC-labeled L. acidophilus NCFM (SlpA+) or SlpA knockout NCK1377CI (SlpB+) for 45 min at 37 °C followed by FACScan analysis. The DC-SIGN-binding specificity was determined by measuring of the bacterial attachment (percentage positive CHO or CHO–DC-SIGN cells) in the presence of blocking antibody against DC-SIGN, D1 (AZN-D1), and EGTA. The error bars represent standard deviation of data from 3 independent experiments; *, P < 0.05.
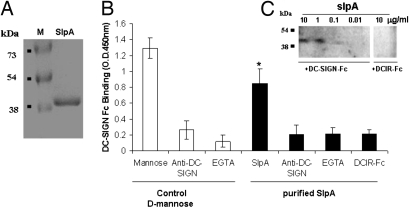
To identify the specific DC-SIGN ligand expressed on the L. acidophilus NCFM cell surface, we took advantage of a number of L. acidophilus NCFM-knockout mutants that were generated (Table S1 and ref. 18). When the various mutants were examined for binding to DC-SIGN-Fc and compared with the wild type, we observed that binding to DC-SIGN-Fc was significantly affected only in the case of the SlpA-knockout strain, NCK1377-CI. This derivative no longer expresses SlpA because of a chromosomal inversion the predominant surface layer protein expressed is SlpB (ref. 19 and S.L. and T.R.K., unpublished data). Fig. 2 shows DC-SIGN-binding data of NCFM (SlpA+) against NCK1377-CI (SlpB+). Moreover, compared with L. acidophilus NCFM, L. acidophilus NCK1377-CI binding was significantly lower to CHO cells transfected with DC-SIGN and uninfluenced by the addition of the blocking antibody or EGTA (Fig. 3). These data led us to the hypothesis that SlpA is a ligand for DC-SIGN. Therefore, we purified SlpA from L. acidophilus NCFM and confirmed that indeed DC-SIGN-Fc specifically ligated to the purified SlpA and that this could be blocked by the addition of anti-DC-SIGN Ab and EGTA (Fig. 4B). The DC-SIGN FC interaction with SlpA was also assessed by Western blots that demonstrated strong binding with the range of 10 to 1 mg of SlpA/mL and a lack of ligation to the control DCIR-Fc (Fig. 4C). In contrast, the same concentrations of SlpB, isolated from L. acidophilus NCK1377-CI, did not ligate to DC-SIGN-Fc in a Western blotting experiment (data not shown). Because DC-SIGN has specificity for high mannose and fucose, we determined the presence of these carbohydrate moieties on SlpA by using plant lectins, ConA (recognizing high mannose > hybrid-type > biantennary N-glycans), and aleuria aurantia lectin (AAL) (specific for α6 fucose). ConA strongly ligated to the SlpA, implying the presence of specific glycans after the Lactobacillus SlpA purification, whereas AAL showed only a weak binding (Fig. S3).
Fig. 4.
Purified S layer protein ligates to DC-SIGN. After the SlpA purification (A), the protein was assayed for its binds to DC-SIGN-Fc by ELISA (B). The data are expressed as the mean ± SD of 3 experiments. (C) Western blotting results for DC-SIGN-Fc and DCIR-Fc binding to different concentrations of SlpA.
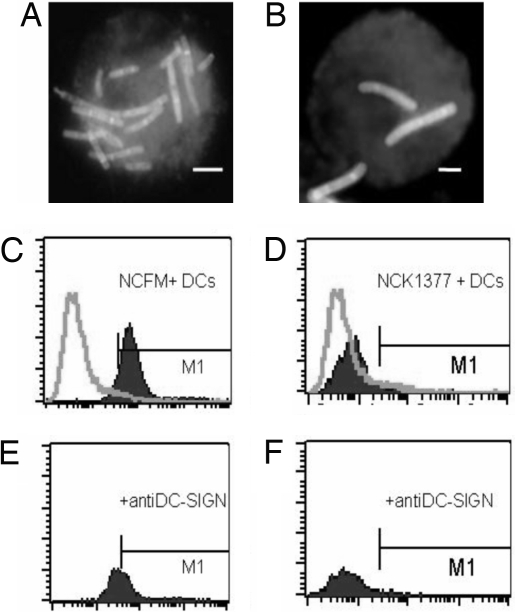
To evaluate the specific interaction of DC-SIGN with SlpA, we compared the binding of NCFM (SlpA-dominant) and NCK1377-CI (SlpB-dominant) to iDCs. DC-SIGN was involved in the capture of L. acidophilus NCFM by the fact that approximately half of the binding L. acidophilus NCFM was abrogated by anti-DC-SIGN Ab at ratio 100:1 (probiotic:iDC) (Fig. 5 C and E). In contrast, attachment of NCK1377-CI (SlpB-dominant) to iDC was significantly lower and unaffected by incubation anti-DC-SIGN Ab (Fig. 5 D and F). The differential DC ligation of NCFM and NCK1377-CI was also supported by visual examination with fluorescent microscopy, and representative images are shown in Fig. 5 A and B, respectively.
Fig. 5.
L. acidophilus NCFM SlpA mediates binding to DC-SIGN on iDCs. (A and B) Microscopic analysis of DCs interacting with fluorescently labeled L. acidophilus NCFM (A) and SlpA-knockout mutant, NCK1377-CI (B). (Scale bars, 5 μm.) (C and D) FACScan analysis of L. acidophilus NCFM or NCK1377-CI binding to iDCs when iDCs (5 × 104 cells) were treated with 5 × 106 FITC-labeled L. acidophilus NCFM (SlpA+) (C) or SlpA-knockout NCK1377-CI (SlpB+) (D) for 45 min at 37 °C. (E and F) Blocking antibodies to DC-SIGN (AZN-D1, c = 20 μg mL−1) were used when iDCs were treated with L. acidophilus NCFM (E) or NCK1377-CI (F). A representative experiment of 4 is shown.
In summary, these findings demonstrate that DCs capture L. acidophilus NCFM via interactions of DC-SIGN and the surface (S) layer protein, SlpA.
Interactions of L. acidophilus NCFM and Its S layer with TLRs-2 and -4.
Next, we evaluated the potential differences of L. acidophilus NCFM and NCK1377-CI to activate TLRs transfected in HEK-293 cells (TLR2 and TLR4 plus MD-2) by determining the level of IL-8 production. PAM3CSK and LPS were analyzed as positive controls for TLR2 and TLR4, respectively. Both NCFM (SlpA dominant) and NCK1377-CI (SlpB dominant) activated the TLR-2 at similar levels and in all ratios examined (1000:1 to 10:1, bacteria:HEK293-TLR2), whereas TLR4 activation was not detected even at the highest doses (Fig. S4). These results indicated that the lack of the S layer protein A expression did not influence the activation of DCs via TLR2 and TLR4.
Enhanced Production of Proinflammatory Cytokines in iDC Treated with L. acidophilus NCK1377 CI Expressing Predominantly SlpB.
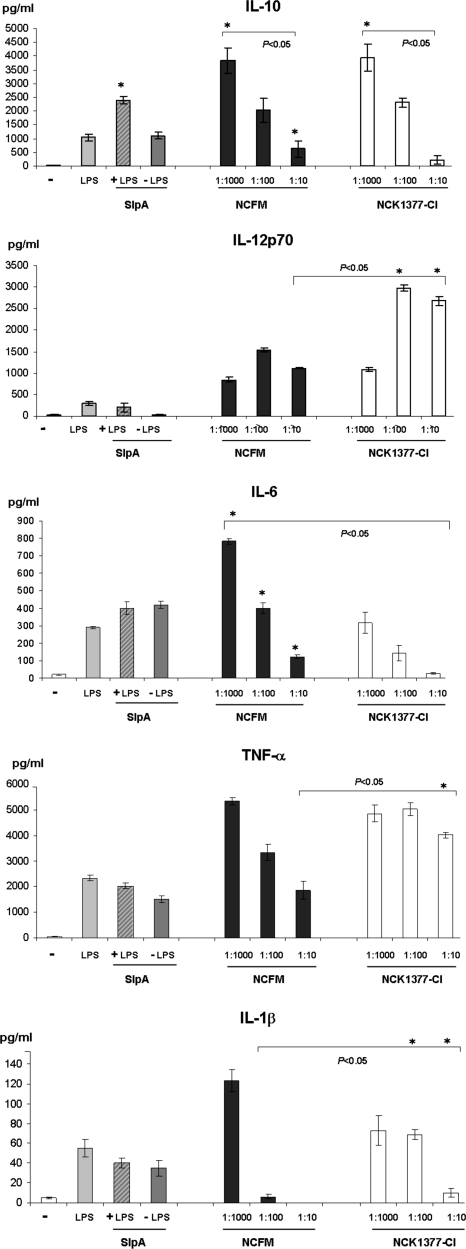
A critical function of DCs to produce cytokines was examined in response to stimulation with both NCFM and NCK1377-CI (SlpB-predominant). The production of the antiinflammatory cytokine IL-10, IL-6, and the proinflammatory cytokines IL-12p70, TNF-α, and IL-1β was assessed at different ratios (bacteria:DC) (Fig. 6). Both strains induced IL-10 at comparable levels in ratios 1,000:1 and 100:1, but the ability of the SlpB-dominant strain to stimulate IL-10 production was reduced significantly at the ratio 10:1 relative to NCFM. To investigate whether SlpA is the structure responsible for the antiinflammatory cytokine profile, we analyzed whether purified SlpA alone or in combination with LPS stimulated higher IL-10 production in iDCs. Indeed, SlpA in combination with LPS induced higher levels of IL-10 compared with LPS or SlpA incubation alone and also led to the production of IL-12p70, TNF-α, IL-1β, and IL-6. Although both bacterial strains (NCFM and NCK1377-CI) were able to stimulate the proinflammatory cytokines such as IL-12p70, TNF-α, and IL-1β at the highest ratio of bacteria to DC (1,000:1), the SlpB-dominant strain was more potent in the induction of these cytokines at the lower ratios of 100:1 or 10:1. Interestingly, incubation of DCs with L. acidophilus NCFM induced a higher IL-6 response compared with the S layer A-knockout at all examined ratios (Fig. 6).
Fig. 6.
L. acidophilus NCFM (SlpA+) and NCK1377-CI (SlpB+) elicit differential iDC cytokine responses. The production of the antiinflammatory cytokines IL-10 and IL-6, and the proinflammatory cytokines IL-12p70, TNF-α, and IL-1β was analyzed by ELISA at different ratios (DCs:NCFM or DCs:NCK1377-CI) or after the iDCs interactions with a purified SlpA (1 μg/mL) with and without LPS (10 ng/mL); −, cytokines produced by iDCs left untreated in those experiments; *, significant difference (P < 0.05) for the cytokines induced at the respective DC:NCFM or DC:NCK1377-CI ratios.
S Layer of L. acidophilus NCFM–DC-SIGN Interaction Is Crucial for the Modulation of T Cell Function.
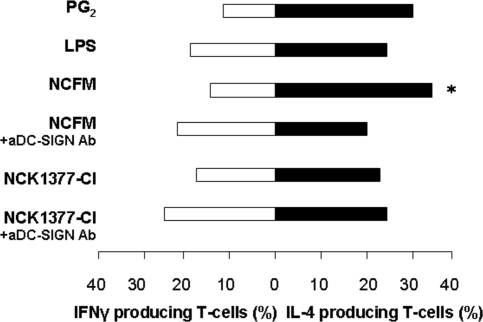
Next we investigated the potential of purified SlpA (1 μg/mL), SlpA combined with LPS, L. acidophilus NCFM (SlpA-dominant), and NCK1377-CI (SlpB-dominant), at ratio of 100:1 (bacteria:DC), to prime T cells responses after their interactions with DC-SIGN on iDCs. Before the incubation of DCs with T cells, the expression of a CD86 maturation marker on DCs was analyzed (Fig. S5). Purified SlpA led to low levels of CD86 on DCs, whereas a higher abundance of CD86 was detected after the interactions between NCFM, NCK1377-CI, and SlpA in combination with LPS. Further, the matured DCs were washed and incubated with autologous naïve CD4+ T cells, as stated by the expression of CD45RA+ on 90% of the CD4+ T cells, and in ratio 5 × 103 DCs added to 20 × 103 naïve T cells, as described in SI Materials and Methods. To examine the DC-SIGN involvement in the activation of T cell differentiation, blocking antibodies to DC-SIGN were also used during the maturation of the DCs in the presence of L. acidophilus NCFM and NCK1377-CI. DCs stimulated with LPS and PGE2 were cocultured with naïve T cells as a positive control. All cocultures of the mature DCs and naïve T cells were performed for up to 15 days, and the resulting T cells were analyzed for their capacity to secrete IFNγ and IL-4, markers for Th1 or Th2 differentiation, respectively. In agreement with previous studies (4, 14), DCs incubated with LPS induce naïve T cell differentiation into a mixed Th1/Th2 response, whereas PGE2 induces a dominant Th2 response (Fig. 7). L. acidophilus NCFM was found to induce more IL-4 compared with IFNγ-producing T cells (34% IL-4 vs. 14% IFNγ, average of n = 4 donors, P < 0.05) (Fig. 7). In comparison, a mixed Th1/Th2 response was characteristic for NCK1377-CI (23% IL-4 vs. 18% IFNγ, n = 4 donors). DCs stimulated with the purified SlpA alone did not induce a T cell proliferation. SlpA combined with LPS maturated DCs led to more IL-4-producing T cells isolated from 2 donors and unpolarized response in the case of T cells from another one (data not shown). Blocking of NCFM ligation to DC-SIGN resulted in a mixed response in T cell differentiation. Cytokine ratios in T cells generated in the presence anti-DC-SIGN Ab and NCK1377-CI was not changed. These results demonstrate that DC-SIGN ligation by the SlpA layer of L. acidophilus NCFM was crucial for the T cells to acquire their differentiation after bacterial stimulation of DCs.
Fig. 7.
L. acidophilus NCFM induces Th2 polarization via C-type DC-SIGN. DCs were maturated with L. acidophilus NCFM and the SlpA-knockout, NCK1377CI-(SlpB+) at a ratio of 100:1 (bacteria:DCs), or SlpA+LPS, in the presence or absence of anti-DC-SIGN Ab (aDC-SIGN). The percentage of IL-4- and INFγ-producing T cells was analyzed upon restimulation of the cultures. The data are the result of 4 independent experiments. *, significant difference (P < 0.05) for the cytokines induced after L. acidophilus NCFM stimulation.
Discussion
In the course of the present work, we demonstrated that the cellular contacts of DCs and L. acidophilus involve interactions between DC-SIGN and SlpA, the dominant protein expressed by the wild-type strain, NCFM. Insertional inactivation of the slpA gene in L. acidophilus NCFM led to a chromosomal inversion (19) and expression of a second S layer protein, SlpB, that was dominant in NCK1377-CI. In addition, the SlpB-dominant mutant up-regulated production of proinflammatory cytokines. Although both the SlpA-dominant parent strain, NCFM, and the SlpB-dominant mutant activated TLR-2 at similar levels, only NCFM-expressing SlpA was captured to DC-SIGN on DCs, an interaction that appeared to be crucial for the activating of IL-4-producing T cells.
Previous studies have showed that DC-SIGN could play an important role in determining the DC maturation and the outcome of the Th1/Th2 balance. Various bacteria can bind DC-SIGN, including H. pylori and mycobacteria (12, 13). Interestingly, ligation of DC-SIGN by mycobacteria and its constituent mannosilated lipoarabinomannan (ManLAM) inhibits LPS-induced DCs maturation and induces IL-10 production (13), whereas H. pylori does not inhibit DCs maturation but does induce increased levels of IL-10 in human DCs (12). N. meningitidis-expressing lgtB LPS also targets DC-SIGN and skewed T cell responses driven by DCs toward Th1 differentiation (15). DC-SIGN could recognize uncharacterized ligands of some commensal bacteria, such as L. reuteri and L. casei, which, unlike the pathogenic species, neither inhibit DC maturation nor induce increased levels of IL-10 even at high concentrations (4). Although the role of DC-SIGN was not studied extensively in many reports on probiotics, our results have both similarities and differences to previous studies on lactobacilli and human DC interactions (5, 16, 17, 20, 21). In line with the current work, human DCs exposed to lactobacilli, including L. acidophilus NCFM, increased CD86 and other costimulatory, adhesion, and activation molecules and induced the expression of IL-10, IL-6, and IL-12 (5, 16). The cytokine profiles of DCs interacting with L. acidophilus NCFM and the SlpB-dominant mutant reported here were concentration-dependent. The study clearly demonstrated that IL-10 was up-regulated compared with IL-12p70 at L. acidophilus NCFM:DCs ratios of 1,000:1 and 100:1. This implies that a dense L. acidophilis NCFM culture is able to stimulate DCs to produce high levels of immune-regulatory IL-10. In contrast, the SlpB-dominant mutant was a potent inducer of proinflammatory cytokines (IL-12p70, TNFα, and IL1β) at lower bacteria:DC ratios (100:1 and/or 10:1). Furthermore, treating DCs with L. acidophilus NCFM (SlpA-dominant) abrogated the binding to DC-SIGN by anti-DC-SIGN Ab to 50% at a ratio of 1:100 (Fig. 5). This suggests that other cell surface proteins might also be involved in the NCFM–DC contact. Nevertheless, the functional analyses of the SlpA-knockout mutant and the DC-SIGN-blocking studies have demonstrated that SlpA interaction with DC-SIGN is crucial for the DC cytokine profiles (high IL-10 and IL-6) and the IL-4 expression by T cells. It remains elusive to what extent L. acidophilus NCFM-stimulated DCs, which produce high levels of IL-10 and IL-6, are capable of skewing T cells toward Th17 or Treg cells. Moreover, because IL-10 expression is considered a key cytokine for maintaining gut homeostasis and partially responsible for the antiinflammatory effects of probiotic cultures (5), the dose effect of probiotic bacteria in treatment of inflammatory diseases, such as allergy, remains to be established. Our work also suggests that the potential switch between SlpA and SlpB might lead to differential immune responses impacting the gut immune homeostasis. Therefore, further studies need to address at what extent the ratio of SlpA to SlpB present on the surface of different intestinal lactic acid bacteria could influence inflammatory conditions in the GI tract.
The immunoregulatory role of DCs is believed to be determined by ligation of pathogen-recognition receptors such as TLRs and CLRs, and signaling pathways induced by these receptors, which can interconnect through a so-called cross-talk (10, 22). In the present work, the purified SlpA induced an IL-10 production and a low expression of CD86 in DCs, and these DCs were unable to induce a T cell proliferation. This supports the notion that at the given concentration (1 μg/mL), the SlpA binding to DC-SIGN is not sufficient to induce a strong maturation in DCs. When SlpA was combined with the TLR4 agonist (LPS), however, a higher IL-10 production by DCs, compared with LPS alone, was observed. A similar effect has been described for ManLAM interactions with DC-SIGN (12), indicating that SlpA binding to DC-SIGN could generate an intracellular signal that interferes with the TLR4-mediated DCs activation. Moreover, both the SlpB-dominant mutant and the parent strain stimulated TLR2 to a similar degree, suggesting that the lack of SlpA expression did not influence the L. acidophilus NCFM activation of TLR2, which senses peptidoglycan structures conserved among lactobacilli (5, 20). It can be further speculated that the DC-SIGN pathway induced at high bacterial doses overruled the generalized TLR2 immune activation, which prevails at low bacterial loads.
The function of the S layer in L. acidophilus NCFM is unknown, but it has been suggested to be important for Lactobacillus adhesion to intestinal epithelial cells and extracellular matrix components (18, 23). The S layers of L. acidophilus and related species are composed of 1 or more proteins of ≈45 kDa. The carbohydrate moieties interacting with different S layer proteins are currently under investigation and may identify a novel carbohydrate ligand for DC-SIGN.
This work does not provide information on the antigen specificity of the induced T cell population. The in vitro experiments tested Lactobacillus-driven DCs maturation and T cell differentiation in polyclonal models by using alloantigen-driven naïve T cell responses and not in an antigen-specific model. This is because of reported difficulties in obtaining sufficient numbers of human antigen-specific, naïve T cells for further analysis (4). It also remains to be established to what extent DCs primed with lactobacilli drive T cell differentiation that are strain-specific and how the bacteria-modulated DCs, as described here, could be generated in different compartments of the intestine.
L. acidophilus NCFM has been used for >30 years as a probiotic culture in various dietary supplements and dairy-based foods, predominately yogurt. In vivo, the strain was found to exert antibody- and cell-mediated responses to Candida albicans in immunodeficient mice, to play a role in decreasing the severity of candidiasis (2), and to elicit morphine-like effects in the GI tract of mice (24). Probiotic treatment with L. acidophilus NCFM also stimulates IL-10 regulatory cytokine expression in the colon and is effective in preventing GI colitis (25). Recently, the DC-SIGN pathway has been demonstrated to be involved in regulation of adaptive immunity by DCs to bacterial, fungal, and viral pathogens (22). The current work establishes that the SlpA-dominant L. acidophilus NCFM interacts with a major receptor on DCs and regulates DC immune functions. It suggests that this probiotic bacterium could directly or indirectly interfere with pathogen-induced effects on the host immune system. These data establish a working hypothesis on the mode of action of probiotic cultures that will guide further investigations into the mechanisms by which these bacteria impact GI states.
Materials and Methods
Bacterial Strains.
The bacterial strains used in this study are identified in Table S1. All strains were obtained from the stock culture collection maintained in the Department of Food Science at North Carolina State University, Raleigh. Bacterial stock cultures were stored at −20 °C in MRS broth (Difco Laboratories) containing 10% (vol/vol) glycerol. All strains were propagated in MRS broth at 37 °C for 18 h, harvested by centrifugation, washed with PBS (50 mL), and added to iDCs. The number of L. acidophilus NCFM was determined by using a published conversation factor (1.2 × 108 cfu/mL per A600 unit) (26).
Extraction and Purification of S Layer Protein A and B.
SlpA from L. acidophilus NCFM and SlpB from L. acidophilus NCK1377-CI were extracted and purified from 100-mL cultures propagated at 37 °C by using a method described in SI Materials and Methods. In short, the bacterial pellets were washed with cold distilled water and suspended in 5 M LiCl at 4 °C followed by stirring for 15 min. The supernatants (containing S layers) were harvested after centrifugation and dialyzed against distilled water. After centrifugation and additional washing step, the S layers were stored at −20 °C before further use.
ELISA and Western Blotting.
L. acidophilus NCFM (A600 of 0.1), its mutants (Table S1), and the purified SlpA (5 μg mL−1) or d-mannose (Sigma–Aldrich) were coated on NUNC maxisorb plates (Roskilde) overnight at room temperature. Plates were first blocked with 1% BSA, and then DC-SIGN-Fc or DCIR-Fc (1 μg mL−1) or the biotinylated plant lectins ConA or AAL (Sigma–Aldrich) were added for 2 h at room temperature in the presence or absence of 10 mM EGTA or 20 μg mL−1 AZN-D1 (27). DC-SIGN-Fc binding was detected by using a goat anti-human conjugated with peroxidase (Jackson Immunoresearch), whereas the ligation of the biotinylated lectins was assessed by using peroxidase-labeled avidin (Vector Laboratories). Before Western blotting using DC-SIGN-Fc or DCIR-Fc, varying amounts of purified SlpA or SlpB (10, 1, 0.1, and 0.01 μg/mL) were electrophoresed on a SDS/polyacrylamide gel followed by blotting to a PVDF membrane (Millipore), as described in ref. 28.
DC Isolation and CHO-DC-SIGN Culturing.
iDCs were obtained from buffy coats of healthy donors (Sanquin), as described elsewhere (29). In short, human peripheral blood mononuclear cells were isolated by a Ficoll gradient. Monocytes were isolated by CD14 magnetic microbeads (MACS; Miltenyibiotec) and differentiated into iDCs in the presence of IL-4 and GM-CSF (500 and 800 units/mL, respectively; Biosource). iDCs expressed high levels of major histocompatibility complex classes I and II, CD11b, CD11c, and ICAM-1 and low levels of CD80 and CD86, confirmed by flow cytometry and defined as iDCs. CHO and CHO transfected with DC-SIGN were maintained in RPMI or DMEM (Invitrogen) containing 8–10% FCS.
DC Maturation, Cytokine Measurements, and DC-Driven TH1/TH2 Differentiation.
Immature monocyte-derived DCs (day 6) were stimulated with different concentrations of L. acidophilus NCFM, the SlpA-knockout strain, NCK1377-CI (SlpB-dominant), or the purified S layer preparations from NCFM (SlpA-dominant, 1 μg/mL) in the presence or absence of LPS (10 ng/mL; Salmonella typhosa, Sigma–Aldrich) for 24 h at 37 °C. Cell surface expression of costimulatory molecules CD86 (BD PharMingen) using phycoerythrin-conjugated antibodies was used to determine maturation. Cytokines were measured in the DCs culture supernatants by ELISA with CytoSets ELISA kits (Biosource) for human IL-6, IL-10, TNF-α, and IL-1β according to the manufacturer's instructions. Human IL-12p70 detection was determined as described in ref. 28.
Before the Th1/Th2 differentiation, iDCs were matured for 2 days with L. acidophilus NCFM, NCK1377-CI, and slpA (1 μg/mL) with and without LPS (10 ng/mL). Subsequently, DCs were incubated with autologous CD45RA+/CD4+ T cells (naïve T cells) at a ratio of 1:4 (DCs:T cells). After 15 days and the application of a specific stimulation protocol, the production of IL-4 and INFγ in T cells was determined by FACs. The details of the DC-driven Th1/Th2 differentiation procedure are fully described in ref. 29 and in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Harro M. Timmerman and Ger T. Rijkers for helpful discussions and suggestions on the topic described in the paper. Work at North Carolina State University on L. acidophilus NCFM to generate cell surface protein mutants was supported by the North Carolina Dairy Foundation, Danisco USA, Inc., and Dairy Management Inc. The work on L. acidophilus NCFM–DC-SIGN interactions was partly supported by Senter, an agency of the Dutch Ministry of Economic Affairs, Grant TSGE3109. S.K.S. is supported by Mozaiek Grant 017.001.136 from the Dutch Scientific Organization NWO. S.R.K. is a recipient of Netherlands Genomics Initiative Fellowship 050-72-431.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810305105/DCSupplemental.
References
- 1.Altermann E, et al. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA. 2005;102:3906–3912. doi: 10.1073/pnas.0409188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders ME, Klaenhammer TR. Invited review: The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2001;84:319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet SJ, Dunnen JD, Gringhuis SI, Geijtenbeek TBH, van Kooyk Y. Innate signaling and regulation of dendritic cell immunity. Curr Opin Immunol. 2007;19:435–440. doi: 10.1016/j.coi.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Mohamadzadeh M, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabelitz D, Medzhitov R. Innate immunity cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Underhill DM, Ozinsky A. Toll-like receptors: Key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 8.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 9.Engering A, et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 10.van Kooyk Y, Geijtenbeek TBH. DC-SIGN: Escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 11.Koppel EA, Saeland E, de Cooker DJM, van Kooyk Y, Geijtenbeek TBH. DC-SIGN specifically recognizes Streptococcus pneumoniae serotypes 3 and 14. Immunobiology. 2005;210:203–210. doi: 10.1016/j.imbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Bergman MP, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Liempt E, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–2615. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Steeghs L, et al. Neisseria meningitidis expressing lgtB lipopolysaccharide targets DC-SIGN and modulates dendritic cell function. Cell Microbiol. 2006;8:316–325. doi: 10.1111/j.1462-5822.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 16.Foligne B, et al. A key role of dendritic cells in probiotic functionality. PloS One. 2007;3:313. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braat H, et al. Impact of lactic acid bacteria on dendritic cells from allergic patients in an experimental model of intestinal epithelium. J Mol Med. 2004;82:197–205. doi: 10.1155/2007/71921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck BL, Altermann E, Svingerud T, Klaenhammer TR. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2005;71:8344–8351. doi: 10.1128/AEM.71.12.8344-8351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boot HJ, Kolen CP, Pouwels PH. Interchange of the active and silent S layer protein genes of Lactobacillus acidophilus by inversion of the chromosomal slp segment. Mol Microbiol. 1996;21:799–809. doi: 10.1046/j.1365-2958.1996.401406.x. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson H, Hessle C, Rudin A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun. 2002;70:6688–6696. doi: 10.1128/IAI.70.12.6688-6696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart AL, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gringhuis SI, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Smit E, Jager D, Martinez B, Tielen FJ, Pouwels PH. Structural and functional analysis of the S layer protein crystallisation domain of Lactobacillus acidophilus ATCC 4356: Evidence for protein–protein interaction of 2 subdomains. J Mol Biol. 2002;324:953–964. doi: 10.1016/s0022-2836(02)01135-x. [DOI] [PubMed] [Google Scholar]
- 24.Rousseaux C, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res. 2005;58:1185–1191. doi: 10.1203/01.pdr.0000183660.39116.83. [DOI] [PubMed] [Google Scholar]
- 26.Greene JD, Klaenhammer TR. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environ Microbiol. 1994:4487–4494. doi: 10.1128/aem.60.12.4487-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geijtenbeek TBH, et al. DC-SIGN–ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 28.van Gisbergen KPJM, Sanchez-Hernandez M, Geijtenbeek TBH, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down-regulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.