Abstract
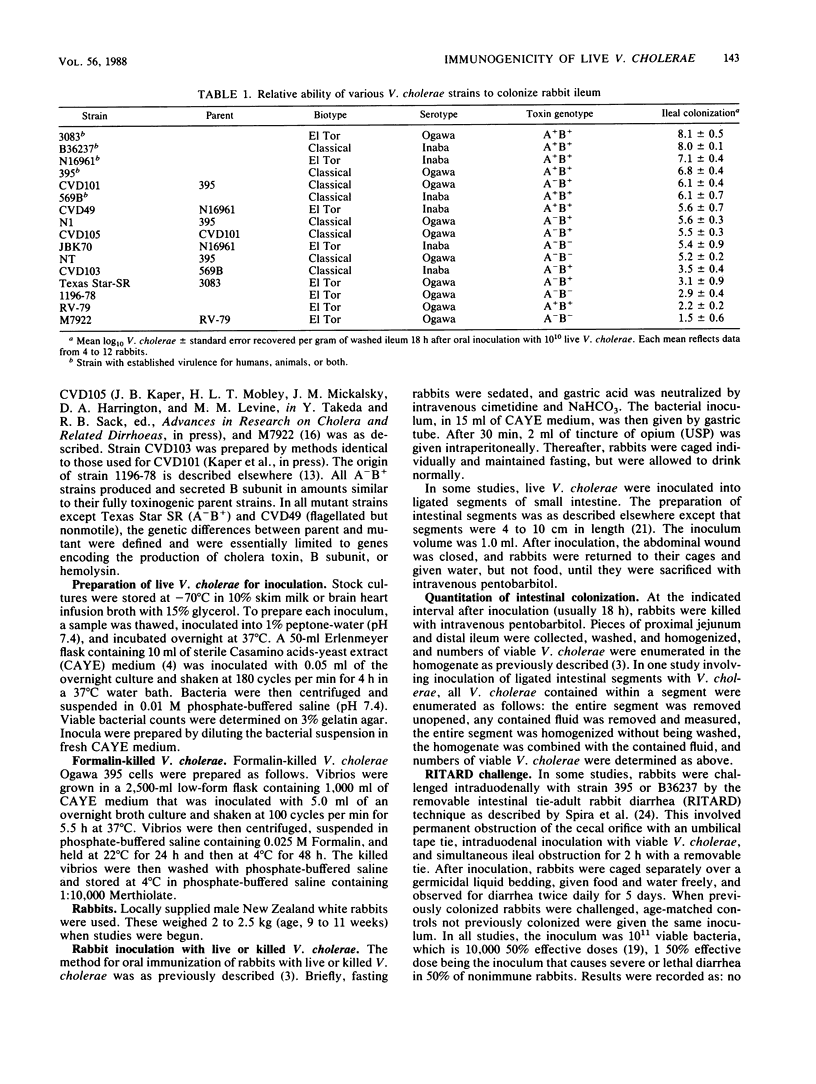
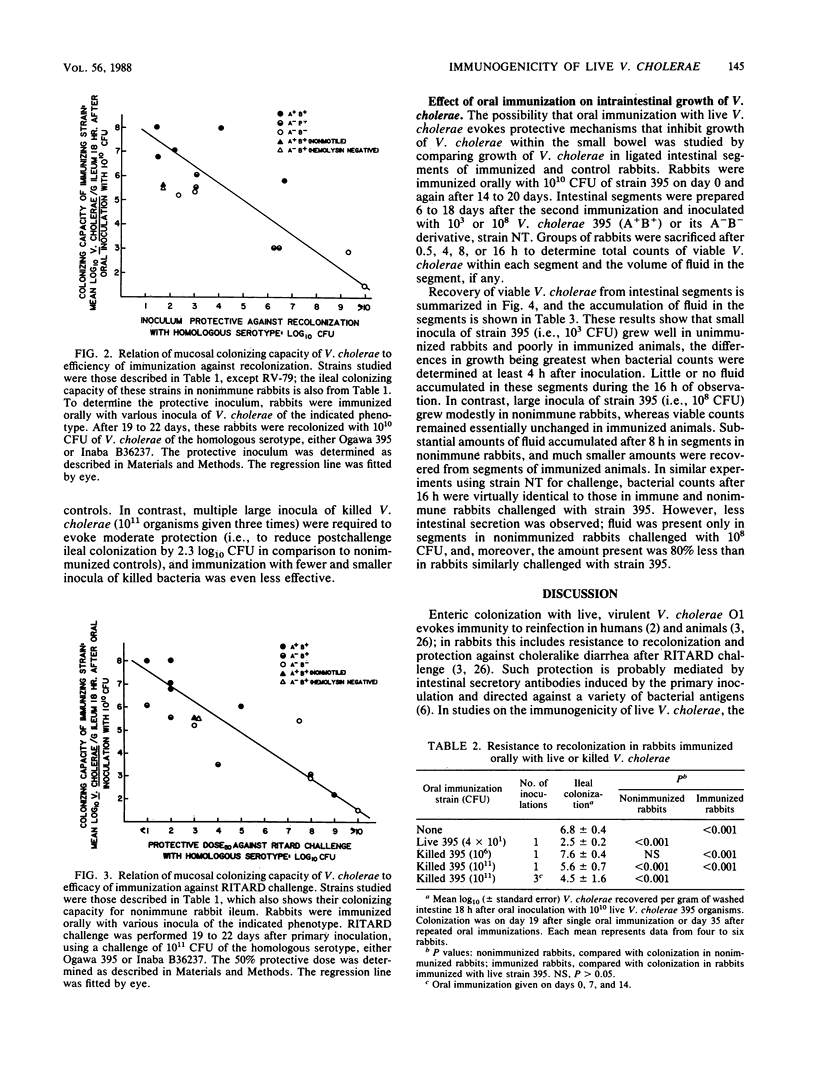
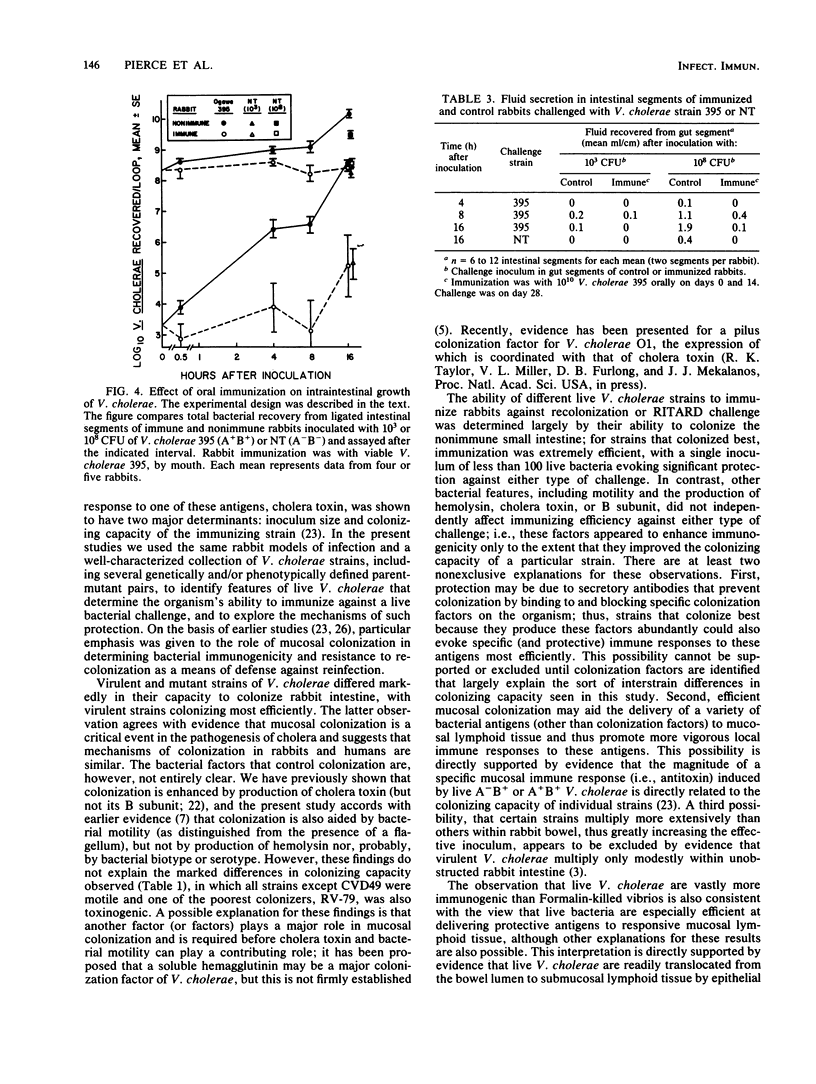
The colonizing capacities of 16 Vibrio cholerae strains, including nine genetically and/or phenotypically defined parent-mutant pairs, were determined in unobstructed adult rabbit small bowel. There were marked interstrain differences in colonizing capacity, which was enhanced by bacterial motility and the production of cholera holotoxin but was unrelated to production of cholera toxin B-subunit or hemolysin or to bacterial serotype or biotype. The role of colonizing capacity and other bacterial features in determining the immunizing efficiency of live V. cholerae was studied by determining the efficiency with which graded inocula of each strain immunized against attempted recolonization of the ileum or induction of choleralike diarrhea by the RITARD (removable intestinal tie-adult rabbit diarrhea) challenge technique using standard inocula of virulent V. cholerae. Mucosal colonizing capacity was the only quantitative predictor of bacterial immunizing capacity; none of the other bacterial features cited above influenced bacterial immunogenicity against either type of challenge, except as they affected colonizing capacity. Live V. cholerae immunized much more efficiently than Formalin-killed bacteria; the former caused marked protection after a single inoculum of 10(2) CFU, whereas the latter gave only partial protection after three inoculations of 10(11) killed organisms. Protection induced by live bacteria was due largely to resistance to colonization and included marked inhibition of bacterial growth within the bowel lumen. These findings strongly suggest that an optimally efficient oral cholera vaccine would be composed of avirulent live V. cholerae selected for their capacity to colonize the small-bowel mucosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. E., Levine M. M., Clements M. L., Young C. R., Svennerholm A. M., Holmgren J. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987 May;55(5):1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Cray W. C., Jr, Tokunaga E., Pierce N. F. Successful colonization and immunization of adult rabbits by oral inoculation with Vibrio cholerae O1. Infect Immun. 1983 Aug;41(2):735–741. doi: 10.1128/iai.41.2.735-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Knop J., Bellamy J. E. Suppression of growth of Vibrio cholerae in the intestine of immunized mice. Med Microbiol Immunol. 1976 Jun 1;162(2):153–158. doi: 10.1007/BF02121325. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Saah A., Nalin D. R., Gill D. M., Craig J. P., Young C. R., Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982 Mar;145(3):296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Moseley S. L., Murphy J. R., Falkow S. Isolation of enterotoxin structural gene deletion mutations in Vibrio cholerae induced by two mutagenic vibriophages. Proc Natl Acad Sci U S A. 1982 Jan;79(1):151–155. doi: 10.1073/pnas.79.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr Oral immunization of dogs with purified cholera toxin, crude cholera toxin, or B subunit: evidence for synergistic protection by antitoxic and antibacterial mechanisms. Infect Immun. 1982 Aug;37(2):687–694. doi: 10.1128/iai.37.2.687-694.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr, Richardson K. Determinants of the immunogenicity of live virulent and mutant Vibrio cholerae O1 in rabbit intestine. Infect Immun. 1987 Feb;55(2):477–481. doi: 10.1128/iai.55.2.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect Immun. 1985 Dec;50(3):813–816. doi: 10.1128/iai.50.3.813-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga E., Cray W. C., Jr, Pierce N. F. Compared colonizing and immunizing efficiency of toxinogenic (A+ B+) Vibrio cholerae and an A- B+ mutant (Texas Star-SR) studied in adult rabbits. Infect Immun. 1984 May;44(2):364–369. doi: 10.1128/iai.44.2.364-369.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


