Abstract
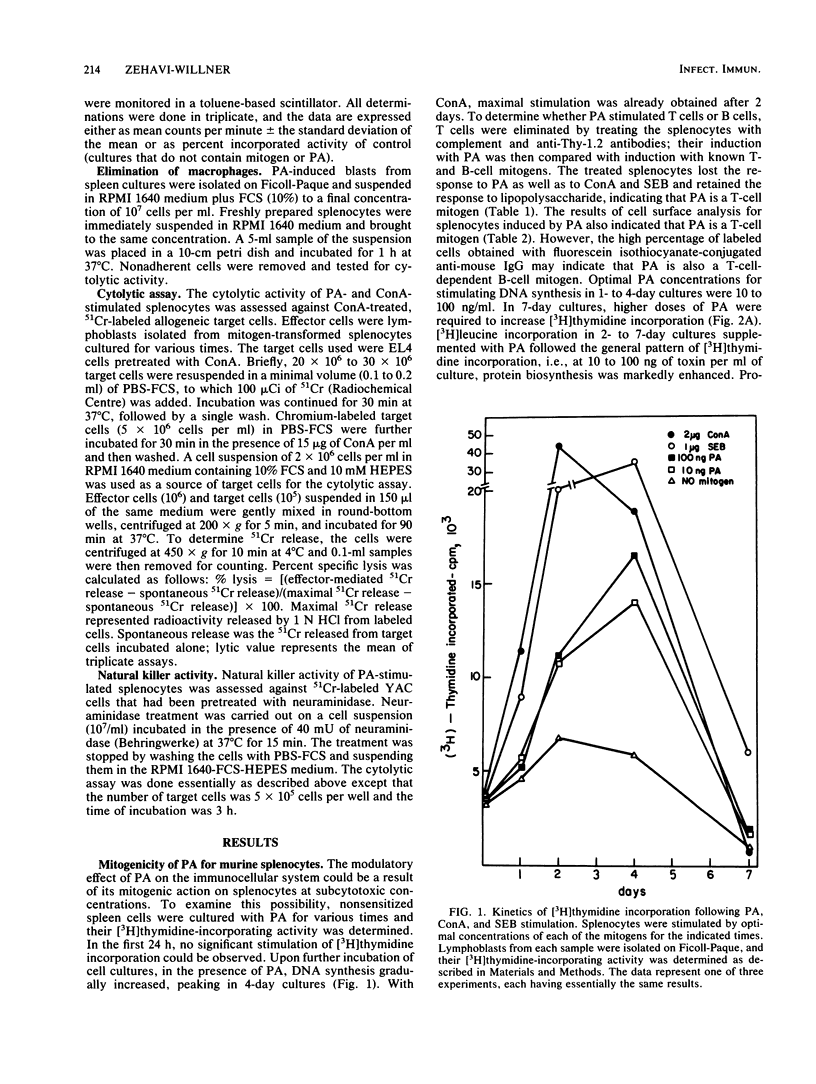
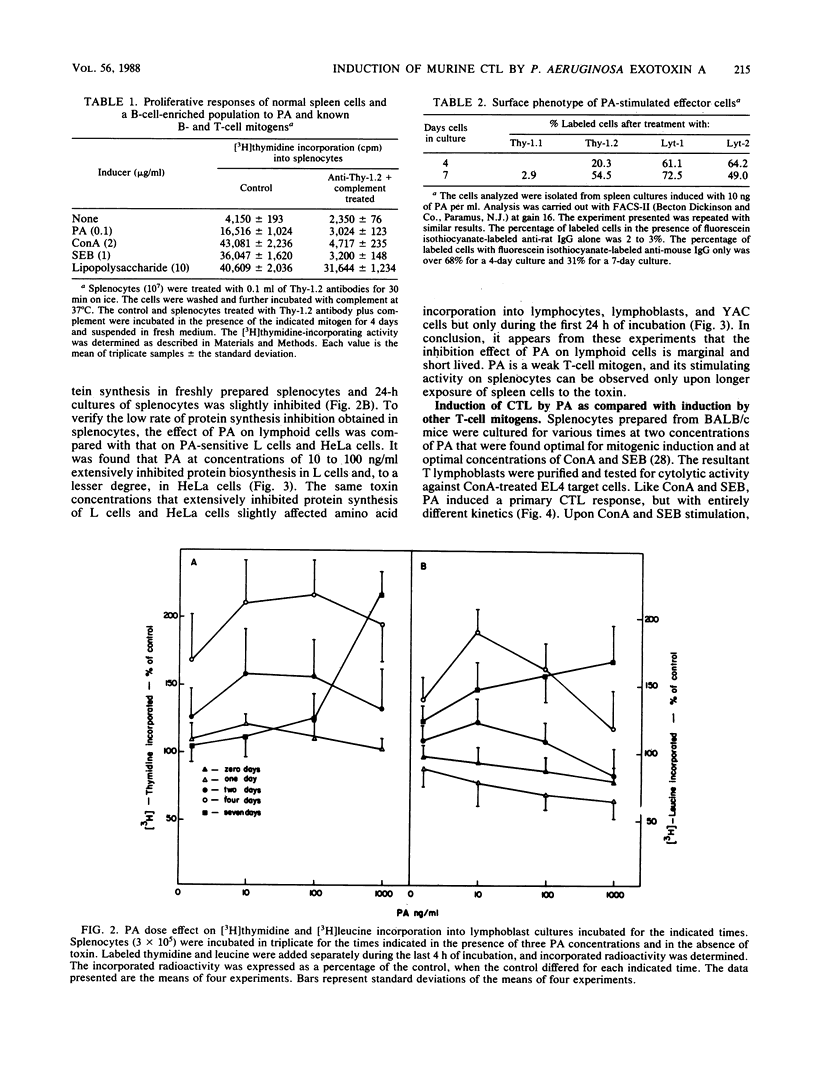
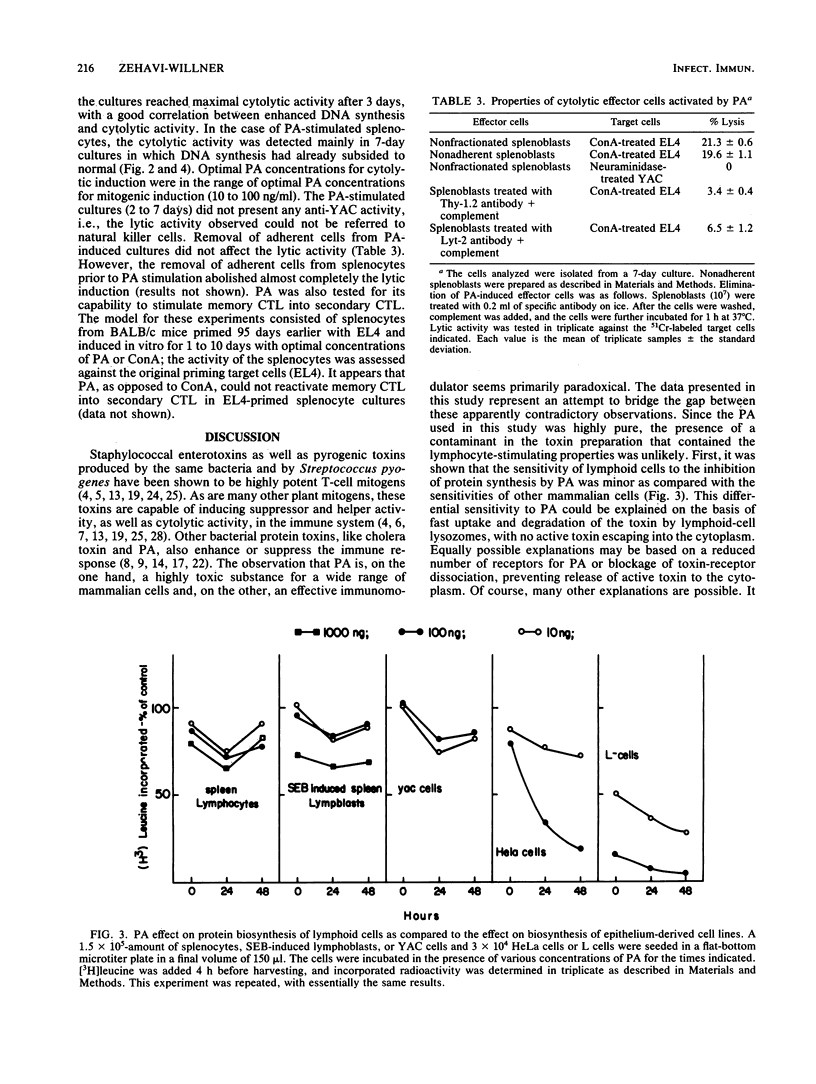
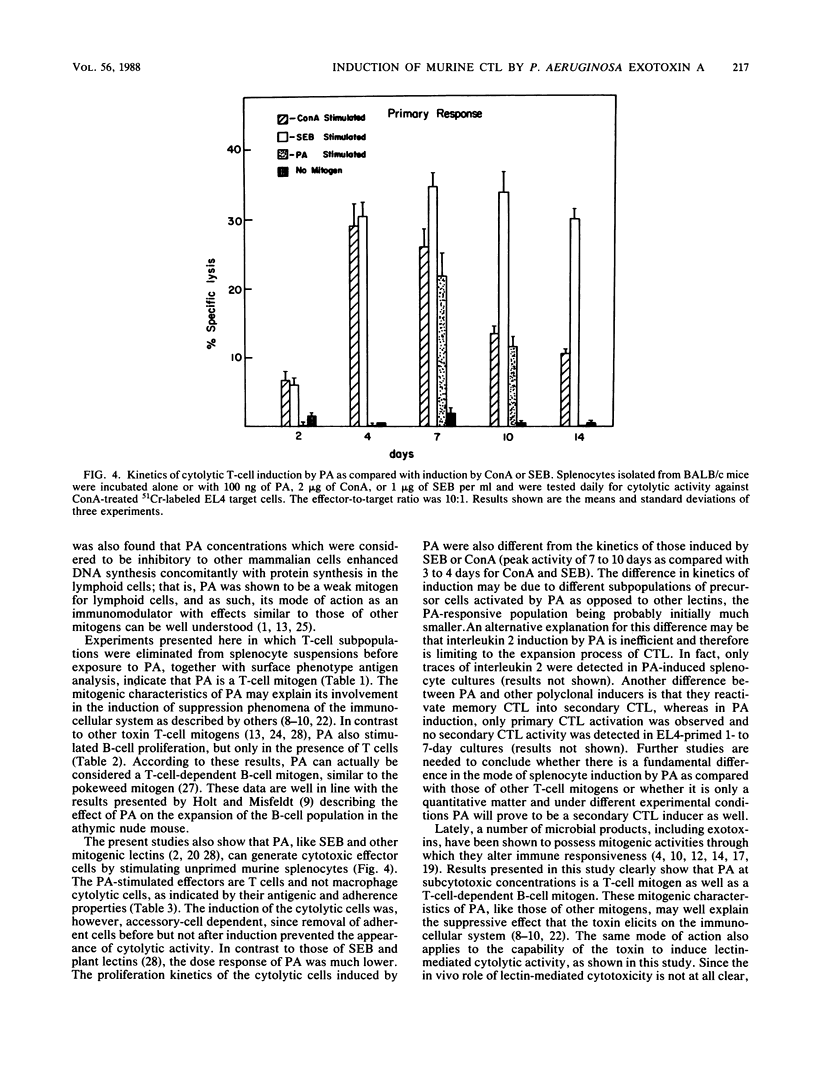
Pseudomonas aeruginosa exotoxin A (PA), a potent protein synthesis inhibitor, was found to be a weak T-cell mitogen for murine splenocytes. Maximal stimulation of [3H]thymidine incorporation was obtained with 10 to 100 ng of toxin per ml following a 4-day induction. PA was also shown to be a polyclonal activator of cytolytic T lymphocytes (CTL), effective against concanavalin A-treated target cells. The effective PA dose for CTL induction was the same as that for mitogenic stimulation, only with a prolonged priming time (7 days). In contrast to other mitogens, PA could not reactivate memory CTL into secondary CTL. The stimulation of CTL by subcytotoxic doses of PA may be relevant to its modulatory effect on the immunocellular system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Clark W. R. An antigen-specific component of lectin-mediated cytotoxicity. Cell Immunol. 1975 Jun;17(2):505–516. doi: 10.1016/s0008-8749(75)80054-2. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. M., Watson D. W. Suppression of antibody response by group A streptococcal pyrogenic exotoxin and characterization of the cells involved. Infect Immun. 1978 Feb;19(2):470–476. doi: 10.1128/iai.19.2.470-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R. P., Rogers T. J. Immunosuppression induced by staphylococcal enterotoxin B. Cell Immunol. 1982 Sep 1;72(1):166–177. doi: 10.1016/0008-8749(82)90294-5. [DOI] [PubMed] [Google Scholar]
- Hale M. L., Hanna E. E. Deregulation of mouse antibody-forming cells by streptococcal pyrogenic exotoxin. II. Modification of spleen T-cell-complemented nude mouse PFC responses. Cell Immunol. 1976 Oct;26(2):168–177. doi: 10.1016/0008-8749(76)90361-0. [DOI] [PubMed] [Google Scholar]
- Hanna E. E., Watson D. W. Host-parasite relationships among group A streptococci. IV. Suppression of antibody response by streptococcal pyrogenic exotoxin. J Bacteriol. 1968 Jan;95(1):14–21. doi: 10.1128/jb.95.1.14-21.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. S., Misfeldt M. L. Alteration of murine immune response by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1984 Jul;45(1):227–233. doi: 10.1128/iai.45.1.227-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. S., Misfeldt M. L. Induction of an immune response in athymic nude mice to thymus-dependent antigens by Pseudomonas aeruginosa exotoxin A. Cell Immunol. 1985 Oct 15;95(2):265–275. doi: 10.1016/0008-8749(85)90314-4. [DOI] [PubMed] [Google Scholar]
- Holt P. S., Misfeldt M. L. Variables which affect suppression of the immune response induced by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1986 Apr;52(1):96–100. doi: 10.1128/iai.52.1.96-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M. Suppression of lymphocyte proliferation by Pseudomonas aeruginosa: mediation by Pseudomonas-activated suppressor monocytes. Infect Immun. 1985 Jun;48(3):832–838. doi: 10.1128/iai.48.3.832-838.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Kateley J. R., Kasarov L., Friedman H. Modulation of in vivo antibody responses by cholera toxin. J Immunol. 1975 Jan;114(1 Pt 1):81–84. [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S. F., Friedman H. Differential effects of cholera toxin pre-treatment on in vitro vs. in vivo immunocyte responses. Proc Soc Exp Biol Med. 1978 Apr;157(4):631–635. doi: 10.3181/00379727-157-40111. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977 Feb;23(2):183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Misfeldt M. L., Hanna E. E. Deregulation of mouse antibody-forming cells by streptococcal pyrogenic exotoxin (SPE). IV. Fractionation of a T-cell subpopulation which generates SPE-induced deregulation of anti-TNP PFC responses. Cell Immunol. 1981 Jan 1;57(1):20–27. doi: 10.1016/0008-8749(81)90116-7. [DOI] [PubMed] [Google Scholar]
- Möller G., Sjöberg O., Andersson J. Mitogen-induced lymphocyte-mediated cytotoxicity in vitro: effect of mitogens selectively activating T or B cells. Eur J Immunol. 1972 Dec;2(6):586–592. doi: 10.1002/eji.1830020621. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Iglewski B. H., Pollack M. Mechanism of action of Pseudomonas aeruginosa exotoxin A in experimental mouse infections: adenosine diphosphate ribosylation of elongation factor 2. Infect Immun. 1978 Jan;19(1):29–33. doi: 10.1128/iai.19.1.29-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Anderson S. E., Jr Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect Immun. 1978 Mar;19(3):1092–1096. doi: 10.1128/iai.19.3.1092-1096.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M. Activation of murine T-suppressor lymphocytes by group A streptococcal and staphylococcal pyurogenic exotoxins. Infect Immun. 1980 Jun;28(3):876–880. doi: 10.1128/iai.28.3.876-880.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. G., Johnson H. M. The effect of staphylococcal enterotoxins on the primary in vitro immune response. J Immunol. 1975 Aug;115(2):575–578. [PubMed] [Google Scholar]
- Stuart R. K., Pollack M. Pseudomonas aeruginosa exotoxin A inhibits proliferation of human bone marrow progenitor cells in vitro. Infect Immun. 1982 Oct;38(1):206–211. doi: 10.1128/iai.38.1.206-211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxdal M. J., Basham T. Y. B and T-cell stimulatory activities of multiple mitogens from pokeweed. Nature. 1974 Sep 13;251(5471):163–164. doi: 10.1038/251163a0. [DOI] [PubMed] [Google Scholar]
- Zehavi-Willner T., Berke G. The mitogenic activity of staphylococcal enterotoxin B (SEB): a monovalent T cell mitogen that stimulates cytolytic T lymphocytes but cannot mediate their lytic interaction. J Immunol. 1986 Oct 15;137(8):2682–2687. [PubMed] [Google Scholar]
- Zehavi-Willner T., Shenberg E., Barnea A. In vivo effect of staphylococcal enterotoxin A on peripheral blood lymphocytes. Infect Immun. 1984 May;44(2):401–405. doi: 10.1128/iai.44.2.401-405.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


