Abstract
Bacteroides loescheii PK1295 fimbriae, which mediate the lactose-sensitive coaggregation with Streptococcus sanguis 34 and the lactose-insensitive coaggregation with Actinomyces israelii PK14, were injected into mice to raise adhesin-specific monoclonal antibodies (MAbs). Supernatants of hybridomas were screened for the capacity to inhibit coaggregation and agglutinate intact bacteria. Of the 10 MAbs that were isolated, 4 were specific and potent inhibitors of the coaggregation between B. loescheii and S. sanguis and two other MAbs specifically inhibited the B. loescheii-A. israelii interaction. None of the six MAbs which inhibited adherence were capable of agglutinating whole cells of B. loescheii, whereas the four remaining MAbs agglutinated whole cells but had no effect on coaggregation. Fab fragments of two MAbs, one that inhibited the coaggregation with S. sanguis and another that inhibited the interaction with A. israelii, also were shown to inhibit the respective coaggregation interactions, suggesting that each of the immunoglobulins recognized its adhesin molecule at or near the active sites. By immunoblotting or immunoprecipitation, the S. sanguis adhesin-specific MAbs reacted with a 75-kilodalton polypeptide present in fimbria-enriched preparations, whereas the A. israelii adhesin-specific MAbs recognized a 45-kilodalton polypeptide in the same preparations. By screening hybridoma supernatants directly for their capacity to block coaggregation, we isolated MAbs which were used to establish that the B. loescheii-S. sanguis and the B. loescheii-A. israelii interactions were mediated by different adhesins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fox P. C., Berenstein E. H., Siraganian R. P. Enhancing the frequency of antigen-specific hybridomas. Eur J Immunol. 1981 May;11(5):431–434. doi: 10.1002/eji.1830110516. [DOI] [PubMed] [Google Scholar]
- Kagermeier A., London J. Identification and preliminary characterization of a lectinlike protein from Capnocytophaga gingivalis (emended). Infect Immun. 1986 Feb;51(2):490–494. doi: 10.1128/iai.51.2.490-494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schifferli D. M., Abraham S. N., Beachey E. H. Use of monoclonal antibodies to probe subunit- and polymer-specific epitopes of 987P fimbriae of Escherichia coli. Infect Immun. 1987 Apr;55(4):923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
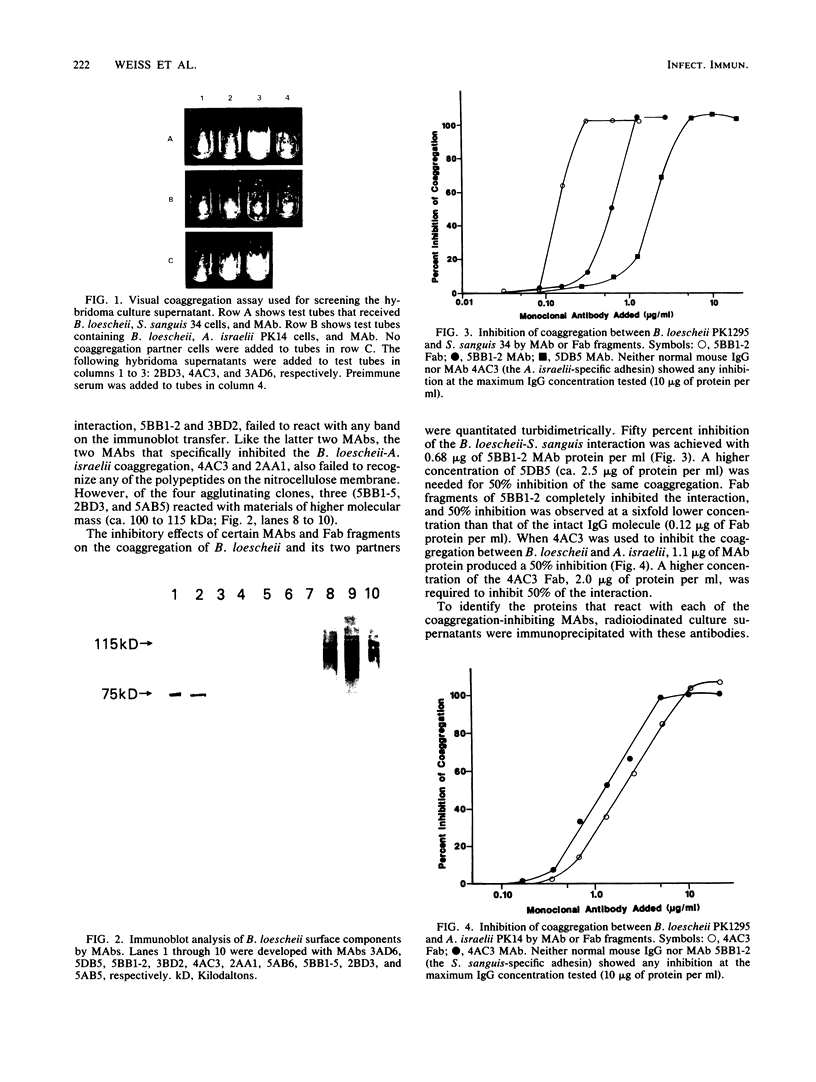
- Weiss E. I., Kolenbrander P. E., London J., Hand A. R., Andersen R. N. Fimbria-associated proteins of Bacteroides loescheii PK1295 mediate intergeneric coaggregations. J Bacteriol. 1987 Sep;169(9):4215–4222. doi: 10.1128/jb.169.9.4215-4222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ree J. M., Schwillens P., van den Bosch J. F. Monoclonal antibodies raised against Pap fimbriae recognize minor component(s) involved in receptor binding. Microb Pathog. 1987 Feb;2(2):113–121. doi: 10.1016/0882-4010(87)90103-3. [DOI] [PubMed] [Google Scholar]