Abstract
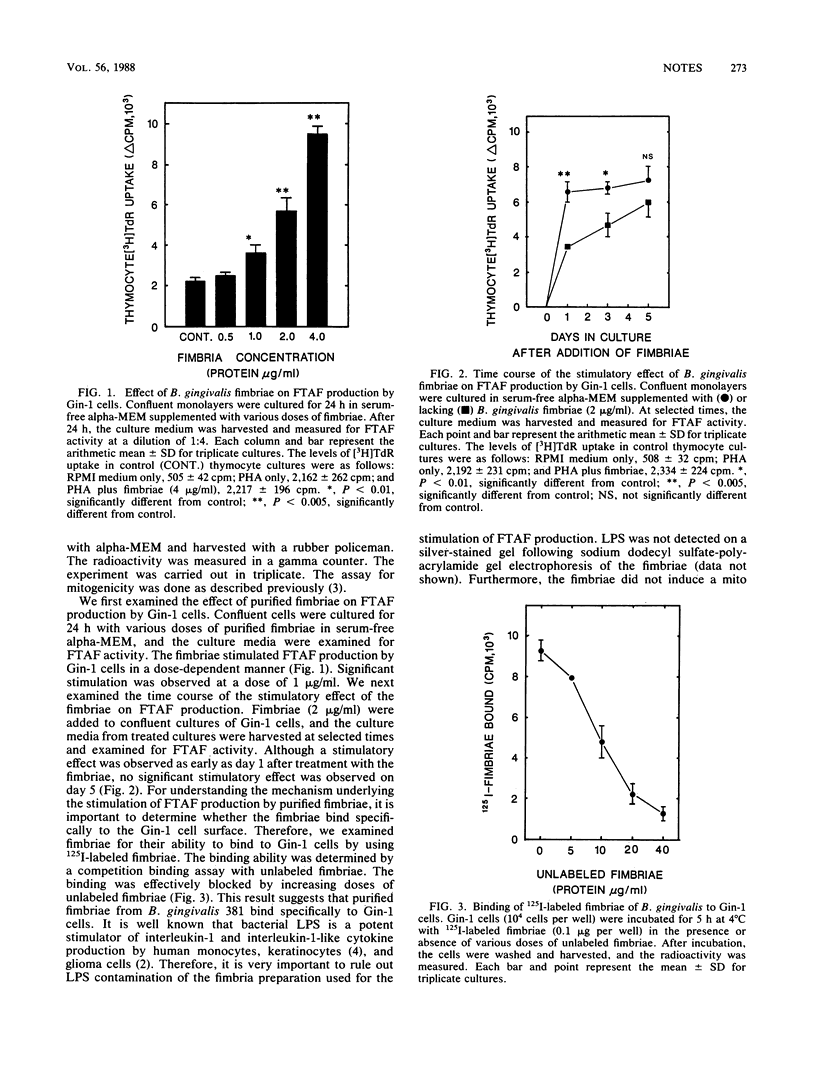
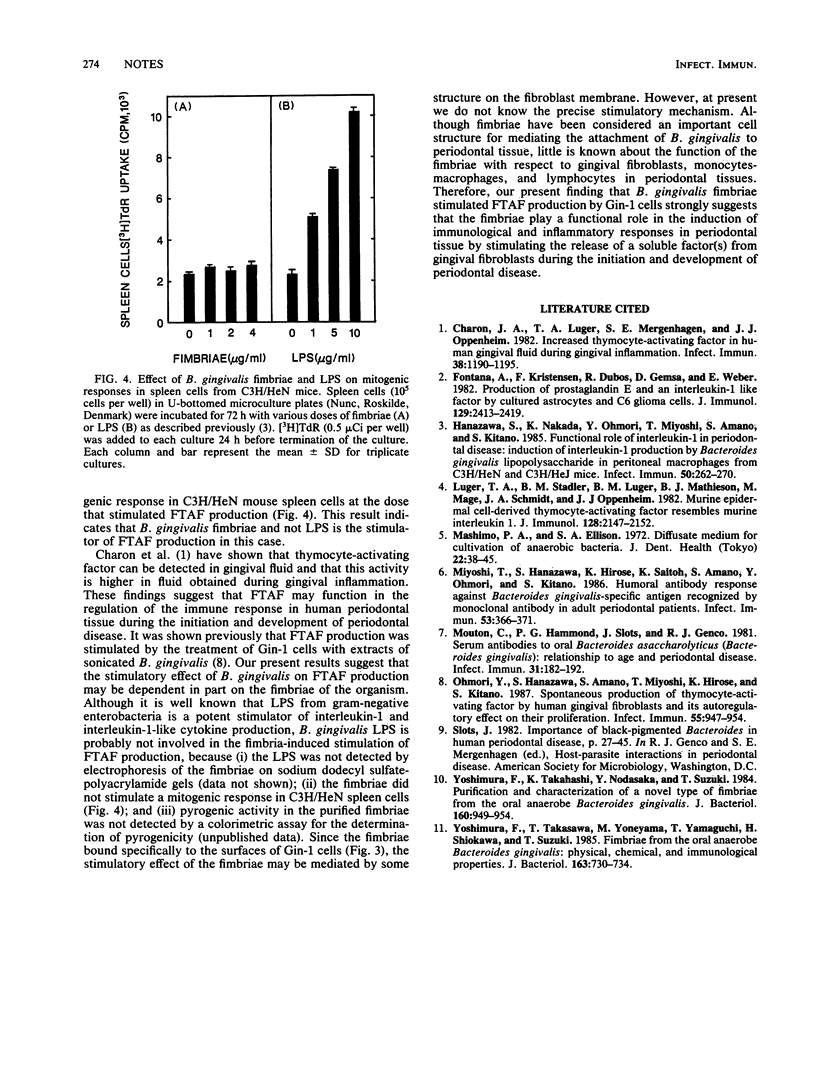
In a previous report (Y. Ohmori, S. Hanazawa, S. Amano, T. Miyoshi, K. Hirose, and S. Kitano, infect. Immun. 55:947-954, 1987), we showed that human gingival fibroblasts spontaneously produce thymocyte-activating factor (FTAF), which stimulates mitogen-induced thymocyte proliferation. In the present study, we examined the effect of Bacteroides gingivalis fimbriae on FTAF production by the cells, because the fimbriae may be involved in attachment of the organism to periodontal tissues. We show here that the fimbriae bind to the cells, which may subsequently lead to the stimulation of FTAF production by the cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charon J. A., Luger T. A., Mergenhagen S. E., Oppenheim J. J. Increased thymocyte-activating factor in human gingival fluid during gingival inflammation. Infect Immun. 1982 Dec;38(3):1190–1195. doi: 10.1128/iai.38.3.1190-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Hanazawa S., Nakada K., Ohmori Y., Miyoshi T., Amano S., Kitano S. Functional role of interleukin 1 in periodontal disease: induction of interleukin 1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect Immun. 1985 Oct;50(1):262–270. doi: 10.1128/iai.50.1.262-270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- Miyoshi T., Hanazawa S., Hirose K., Saitoh K., Amano S., Ohmori Y., Kitano S. Humoral antibody response against Bacteroides gingivalis-specific antigen recognized by monoclonal antibody in adult periodontal patients. Infect Immun. 1986 Aug;53(2):366–371. doi: 10.1128/iai.53.2.366-371.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori Y., Hanazawa S., Amano S., Miyoshi T., Hirose K., Kitano S. Spontaneous production of thymocyte-activating factor by human gingival fibroblasts and its autoregulatory effect on their proliferation. Infect Immun. 1987 Apr;55(4):947–954. doi: 10.1128/iai.55.4.947-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takasawa T., Yoneyama M., Yamaguchi T., Shiokawa H., Suzuki T. Fimbriae from the oral anaerobe Bacteroides gingivalis: physical, chemical, and immunological properties. J Bacteriol. 1985 Aug;163(2):730–734. doi: 10.1128/jb.163.2.730-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


