Abstract
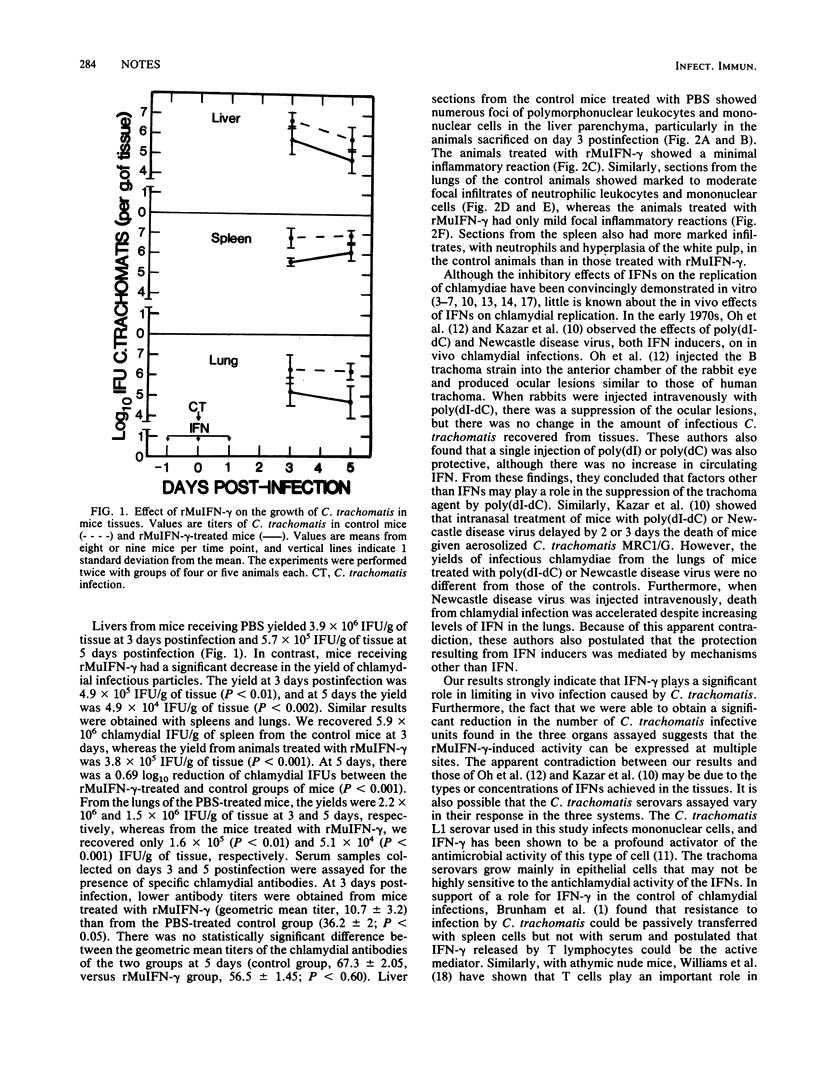
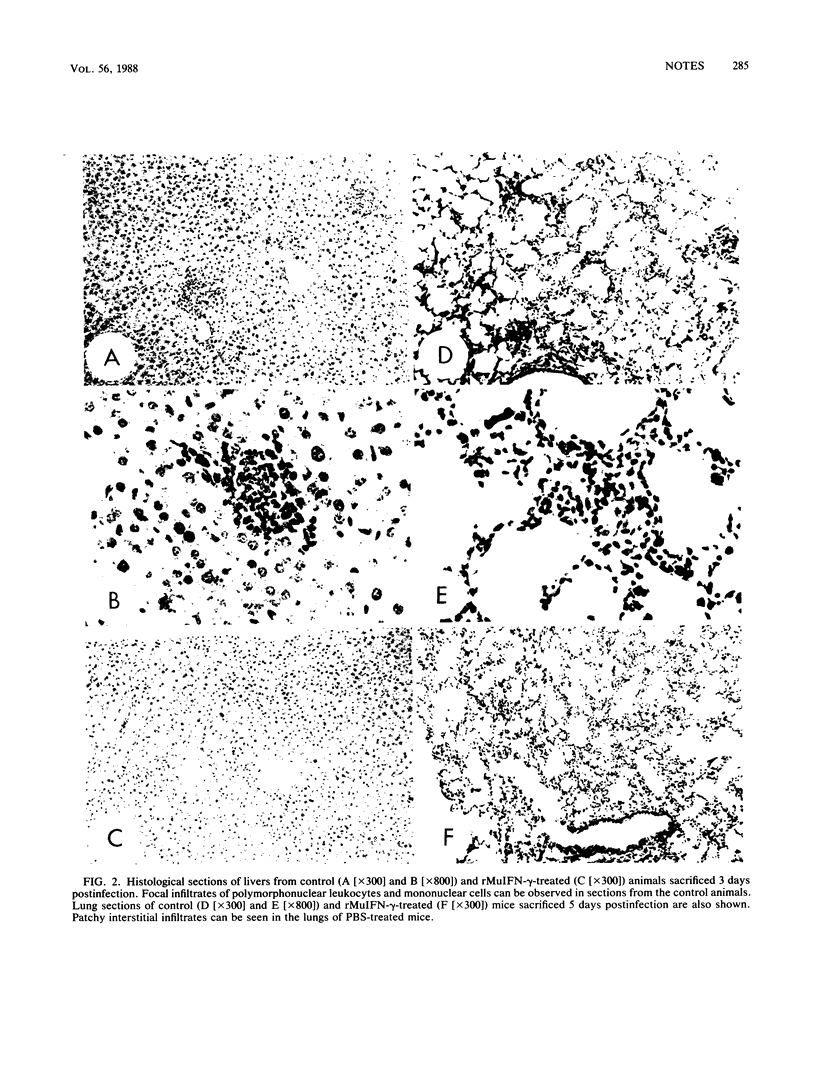
Chlamydia trachomatis serovar L1 injected intravenously in mice resulted in systemic nonlethal infections of the animals. Treatment of mice with recombinant murine gamma interferon resulted in a decrease in the number of infectious C. trachomatis organisms recovered from the lungs, spleens, and livers as well as in a decrease of the inflammatory reaction in those organs when assessed 3 and 5 days after challenge.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunham R. C., Kuo C., Chen W. J. Systemic Chlamydia trachomatis infection in mice: a comparison of lymphogranuloma venereum and trachoma biovars. Infect Immun. 1985 Apr;48(1):78–82. doi: 10.1128/iai.48.1.78-82.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Oh J. O., Ostler H. B., Schachter J. Protective effect of a synthetic polynucleotide complex (poly I: C) on ocular lesions produced by trachoma agent in rabbits. Infect Immun. 1970 Jun;1(6):566–573. doi: 10.1128/iai.1.6.566-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Jaffe E. A., Murray H. W. Oxygen-independent inhibition of intracellular Chlamydia psittaci growth by human monocytes and interferon-gamma-activated macrophages. J Immunol. 1986 Jul 15;137(2):689–692. [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Sueltenfuss E. A., Pollard M. Cytochemical Assay of Interferon Produced by Duck Hepatitis Virus. Science. 1963 Feb 15;139(3555):595–596. doi: 10.1126/science.139.3555.595. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Goebel J. M., Czarniecki C. W., Peterson E. M. Ultrastructural analysis of the growth cycle of Chlamydia trachomatis in mouse cells treated with recombinant human alpha-interferons. Exp Mol Pathol. 1984 Oct;41(2):227–235. doi: 10.1016/0014-4800(84)90039-x. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Goebel J. M., Fennie C. W., Czarniecki C. W. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect Immun. 1985 Mar;47(3):719–722. doi: 10.1128/iai.47.3.719-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]