Abstract
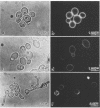
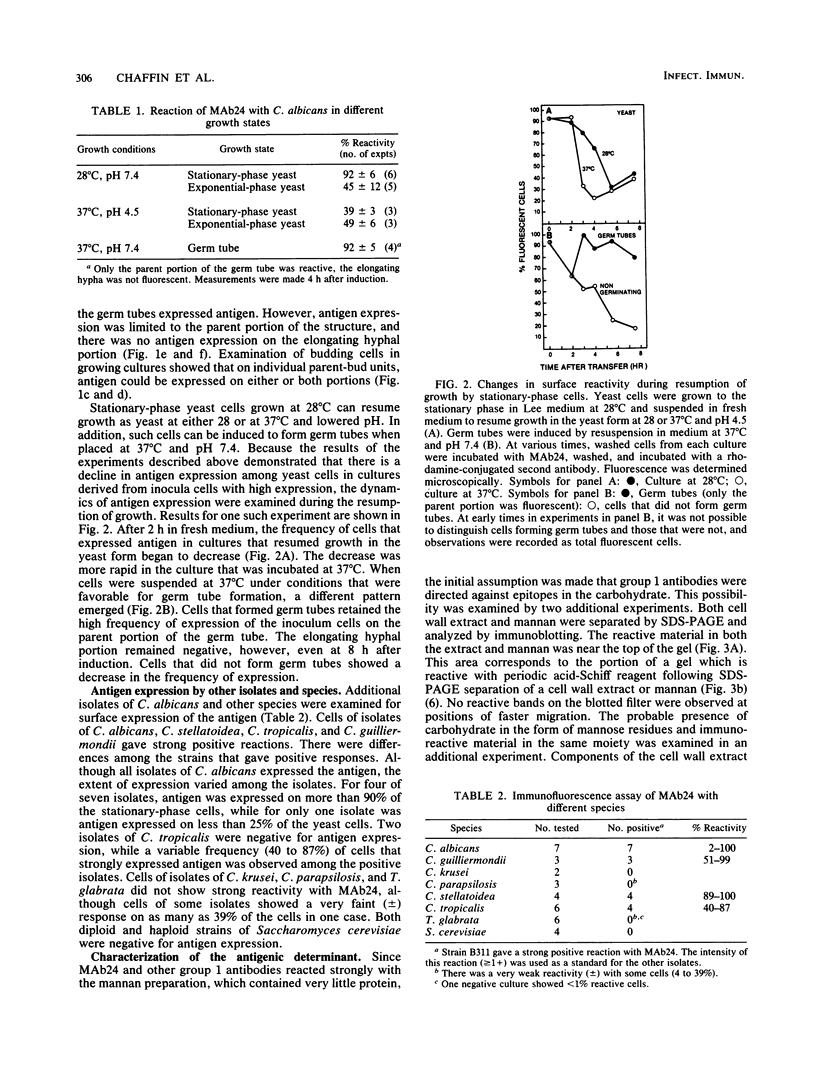
The surface expression of an antigenic determinant that is present in the cell wall of Candida albicans was investigated with monoclonal antibody 24 (MAb24), an immunoglobulin M MAb. The proportion of the cell population that expressed the epitope under different growing conditions was determined by indirect immunofluorescence microscopy. More than 90% of stationary-phase yeast cells of strain B311 grown at 28 degrees C expressed the antigen. Less than 50% of yeast cells grown exponentially at 28 degrees C or either growing or stationary-phase yeast cells cultivated at 37 degrees C expressed the epitope. Germ tubes, which were induced at 37 degrees C from stationary-phase yeast cells grown at 28 degrees C, expressed the determinant on the parent yeast but not the hyphal portion of the germ tube. The change in antigen expression by stationary-phase cells grown at 28 degrees C, when they resumed growth by bud formation, suggested that antigen expression was lost by cells in the inoculum prior to the first cell division. By using the same assay, strong positive reactions were observed in stationary-phase cultures of other isolates of C. albicans, C. guilliermondii, C. stellatoidea, and C. tropicalis, but not with isolates of C. krusei, C. parapsilosis, or Torulopsis glabrata. The identification of the antigenic determinant as a carbohydrate was based on three observations: (i) interaction with a mannan preparation from the same organism, (ii) sensitivity of the antigen to periodate but not proteases, and (iii) coincidence of the migration of antigen during electrophoresis with material which stained intensely with carbohydrate but not with protein reagents. These observations suggest that the expression of the antigenic determinant of MAb24 is dependent on the growth conditions, growth state, and morphology of the cell and that the topography of the cell surface is dynamic.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Ultrastructural and biochemical studies of two dynamically expressed cell surface determinants on Candida albicans. Infect Immun. 1986 Jan;51(1):327–336. doi: 10.1128/iai.51.1.327-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of a cell surface determinant on Candida albicans as evidenced by an agglutinating monoclonal antibody. Infect Immun. 1984 Mar;43(3):966–972. doi: 10.1128/iai.43.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986 Jan;51(1):337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Mattia E., Boldrini L. Agglutination of blastospores of Candida albicans by concanavalin A and its relationship with the distribution of mannan polymers and the ultrastructure of the cell wall. J Gen Microbiol. 1978 Apr;105(2):263–273. doi: 10.1099/00221287-105-2-263. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L. Effect of tunicamycin on germ tube and yeast bud formation in Candida albicans. J Gen Microbiol. 1985 Aug;131(8):1853–1861. doi: 10.1099/00221287-131-8-1853. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Sogin S. J. Germ tube formation from zonal rotor fractions of Candida albicans. J Bacteriol. 1976 May;126(2):771–776. doi: 10.1128/jb.126.2.771-776.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin W. L., Stocco D. M. Cell wall proteins of Candida albicans. Can J Microbiol. 1983 Oct;29(10):1438–1444. doi: 10.1139/m83-220. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L. The relationship between yeast cell size and cell division in Candida albicans. Can J Microbiol. 1984 Feb;30(2):192–203. doi: 10.1139/m84-030. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J Bacteriol. 1961 Oct;82:570–573. doi: 10.1128/jb.82.4.570-573.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hopwood V., Poulain D., Fortier B., Evans G., Vernes A. A monoclonal antibody to a cell wall component of Candida albicans. Infect Immun. 1986 Oct;54(1):222–227. doi: 10.1128/iai.54.1.222-227.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., King R. D. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983 Sep;41(3):1024–1030. doi: 10.1128/iai.41.3.1024-1030.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Li Y. T. Studies on the glycosidases in jack bean meal. I. Isolation and properties of alpha-mannosidase. J Biol Chem. 1967 Dec 10;242(23):5474–5480. [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L. H., Soll D. R. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp Cell Res. 1979 Apr;120(1):167–179. doi: 10.1016/0014-4827(79)90547-0. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Kagaya K., Fukazawa Y., Soe G. Production and characterization of agglutinating monoclonal antibodies against predominant antigenic factors for Candida albicans. J Clin Microbiol. 1986 May;23(5):881–886. doi: 10.1128/jcm.23.5.881-886.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Lefebvre B., Husson M. O. Antigenic variability between Candida albicans blastospores isolated from healthy subjects and patients with Candida infection. Sabouraudia. 1982 Sep;20(3):173–177. [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Vernes A., Popeye R., Biguet J. Antigenic variations of Candida albicans in vivo and in vitro--relationships between P antigens and serotypes. Sabouraudia. 1983 Jun;21(2):99–112. [PubMed] [Google Scholar]
- SUMMERS D. F., GROLLMAN A. P., HASENCLEVER H. F. POLYSACCHARIDE ANTIGENS OF CANDIDA CELL WALL. J Immunol. 1964 Mar;92:491–499. [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Sullivan P. A., Yin C. Y., Molloy C., Templeton M. D., Shepherd M. G. An analysis of the metabolism and cell wall composition of Candida albicans during germ-tube formation. Can J Microbiol. 1983 Nov;29(11):1514–1525. doi: 10.1139/m83-233. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. M., Kenny G. E. Characterization of antigens specific to the surface of germ tubes of Candida albicans by immunofluorescence. Infect Immun. 1984 Mar;43(3):850–855. doi: 10.1128/iai.43.3.850-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Biguet J. Etudes cytochimiques et ultrastructurales de la paroi de Candida albicans. Arch Microbiol. 1979;123(3):245–249. doi: 10.1007/BF00406657. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Herbaut J., Biguet J. Cytochemical and ultrastructural studies of Candida albicans. II. Evidence for a cell wall coat using concanavalin A. J Ultrastruct Res. 1981 Apr;75(1):50–59. doi: 10.1016/s0022-5320(81)80099-8. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Fukazawa Y., Taguchi M., Nakase T., Shinoda T. Serologic aspects on yeast classification. Mycopathol Mycol Appl. 1974 Aug 30;53(1):77–91. doi: 10.1007/BF02127199. [DOI] [PubMed] [Google Scholar]