Abstract
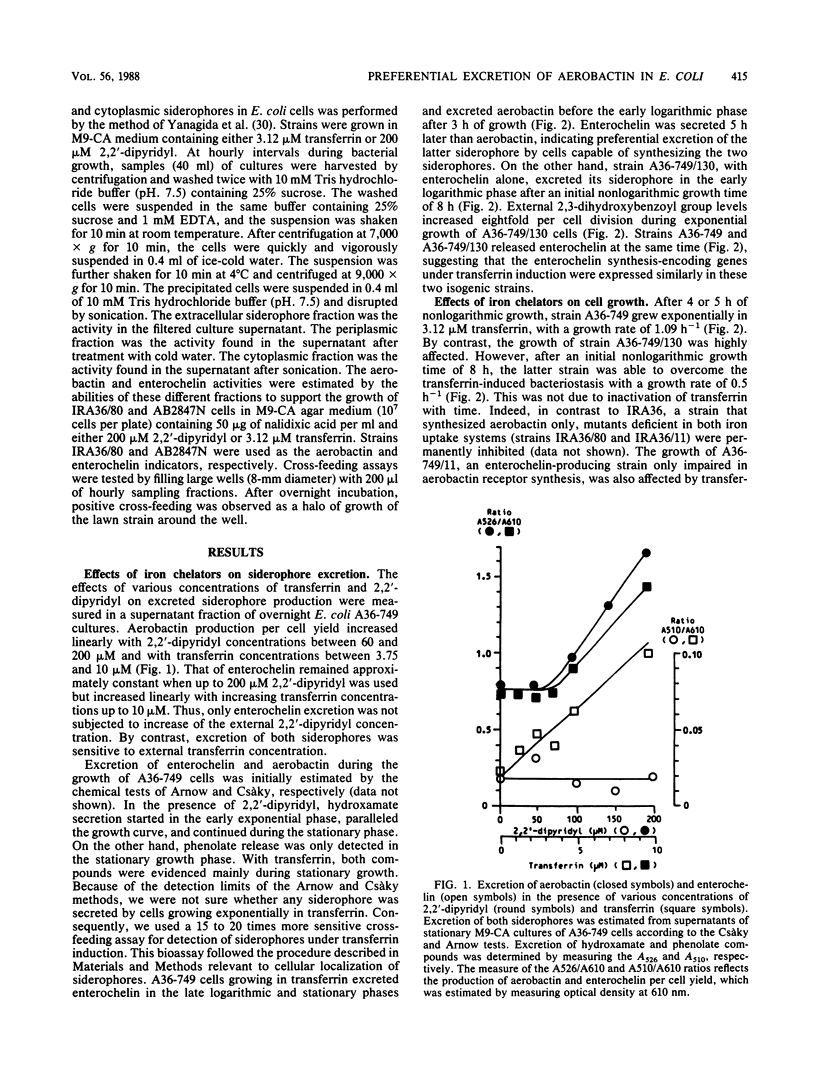
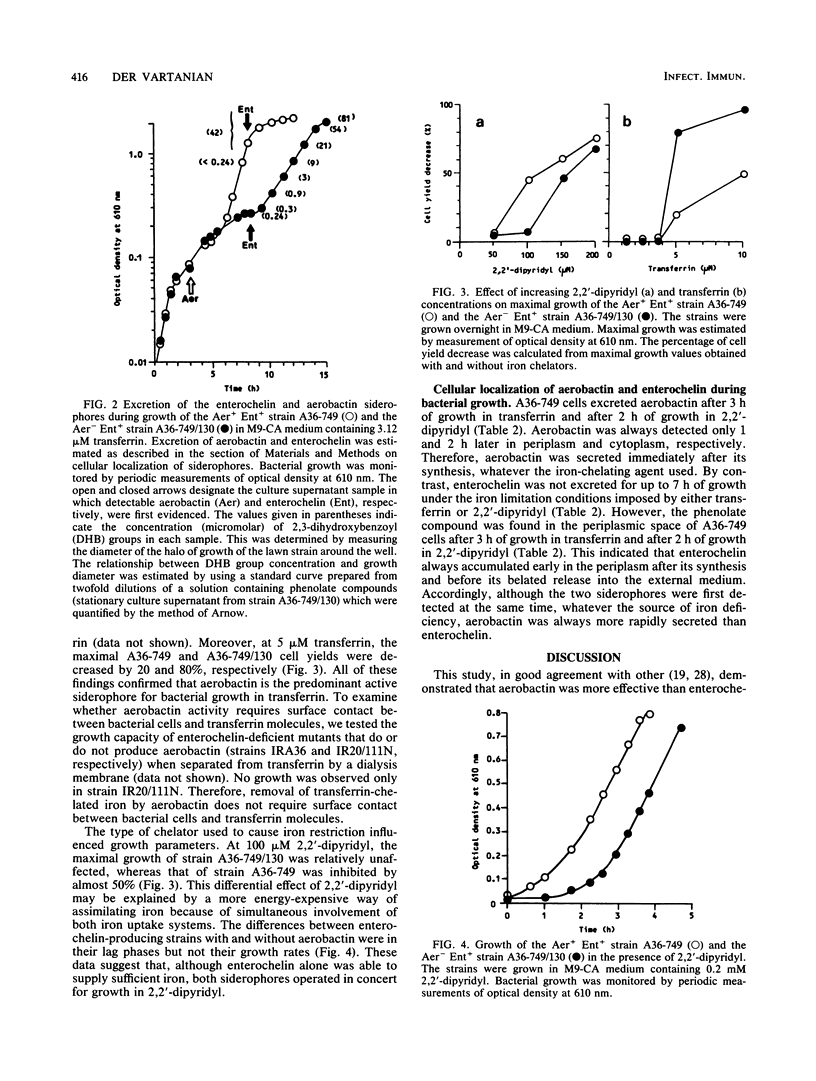
Secretion of aerobactin is thought to play an important part in the virulence of invasive Escherichia coli also capable of synthesizing enterochelin. Why, despite its markedly lower affinity for iron than that of enterochelin, aerobactin proves to be the predominant active siderophore for bacterial growth in transferrin was investigated. We studied the action of two iron chelators, 2,2'-dipyridyl and transferrin, in expression of the aerobactin and enterochelin genes. Specifically, we describe the sequential localization of the two siderophores in the cell compartments during bacterial growth under different iron limitation conditions. Our results demonstrated that, whatever the exogenous iron-chelating agent used, aerobactin was rapidly excreted, whereas enterochelin accumulated early in periplasm before its very belated release into the external medium. This work also showed that the advantage of aerobactin over enterochelin in competition with transferrin was not due to (i) lack of enterochelin activity, (ii) a cell-bound aerobactin-dependent mechanism, (iii) antagonism between the two siderophores, and probably (iv) genetic preferential induction of aerobactin. We propose that the superiority of aerobactin in competing with transferrin for iron(III) was a consequence of its more rapid excretion with respect to enterochelin. In contrast to transferrin, 2,2'-dipyridyl induced a greater efficiency of enterochelin, possibly by a more permanent function as iron-binding compounds in the bacterial envelope. In summary, unlike aerobactin, enterochelin appears to be a weakly secreted high-affinity iron ligand.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Aerobactin genes in clinical isolates of Escherichia coli. J Bacteriol. 1985 Feb;161(2):727–735. doi: 10.1128/jb.161.2.727-735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Burkhardt R. Regulation of the ColV plasmid-determined iron (III)-aerobactin transport system in Escherichia coli. J Bacteriol. 1982 Oct;152(1):223–231. doi: 10.1128/jb.152.1.223-231.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Burkhardt R., Schneider R., Zimmermann L. Chromosomal genes for ColV plasmid-determined iron(III)-aerobactin transport in Escherichia coli. J Bacteriol. 1982 Aug;151(2):553–559. doi: 10.1128/jb.151.2.553-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzoni-Gatel D. Relationship between pathogenicity in footpad lesion test and invasiveness in Escherichia coli. Ann Microbiol (Paris) 1984 Nov-Dec;135B(3):323–329. doi: 10.1016/s0769-2609(84)80099-x. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Buck M., Stevenson P., Griffiths E. Iron regulated outer membrane proteins of Escherichia coli: variations in expression due to the chelator used to restrict the availability of iron. J Gen Microbiol. 1986 May;132(5):1373–1378. doi: 10.1099/00221287-132-5-1373. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Brandt P., Mahoney J. R., Lee J. T., Jr Haptoglobin: a natural bacteriostat. Science. 1982 Feb 5;215(4533):691–693. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Grewal K. K., Warner P. J., Williams P. H. An inducible outer membrane protein involved in aerobactin-mediated iron transport by co1V strains of Escherichia coli. FEBS Lett. 1982 Apr 5;140(1):27–30. doi: 10.1016/0014-5793(82)80513-9. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Heidinger S., Braun V., Pecoraro V. L., Raymond K. N. Iron supply to Escherichia coli by synthetic analogs of enterochelin. J Bacteriol. 1983 Jan;153(1):109–115. doi: 10.1128/jb.153.1.109-115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka K., Bindereif A., Neilands J. B. Aerobactin-mediated utilization of transferrin iron. Biochemistry. 1982 Dec 7;21(25):6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- May P. M., Linder P. W., Williams D. R. Ambivalent effect of protein binding on computed distributions of metal ions complexed by ligands in blood plasma. Experientia. 1976 Dec 15;32(12):1492–1494. doi: 10.1007/BF01924411. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Silver R. P., Crosa J. H. Occurrence of chromosome- or plasmid-mediated aerobactin iron transport systems and hemolysin production among clonal groups of human invasive strains of Escherichia coli K1. Infect Immun. 1986 Apr;52(1):192–199. doi: 10.1128/iai.52.1.192-199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Carbonetti N. H. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect Immun. 1986 Mar;51(3):942–947. doi: 10.1128/iai.51.3.942-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida N., Uozumi T., Beppu T. Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli. J Bacteriol. 1986 Jun;166(3):937–944. doi: 10.1128/jb.166.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


