Abstract
Nicotinamide adenine dinucleotide (NAD+), a precursor of molecules involved in cell regulatory processes, is released in extra-cellular compartments after stress or inflammation.This study investigates the expression in the human cornea of CD38 and CD157, two NAD+-consuming ectoenzymes and surface receptors. The analysis in corneal epithelial and stromal cells was performed by means of multiple approaches, which included immunofluorescence, reverse transcriptase polymerase chain reaction (RT-PCR), Western blot, and confocal microscopy. The presence of enzymatically active NAD+-consumers in intact corneal cells was analyzed by high performance liquid chromatography (HPLC)-based assays. The results obtained show that CD38 and CD157 are expressed constitutively by corneal cells: CD38 appears as a 45-kDa monomer, while CD157 is a 42- to 45-kDa doublet. The molecules are enzymatically active, with features reminiscent of those observed in human leukocytes. CD38 is expressed by cells of the suprabasal limbal epithelium, whereas it is not detectable in central corneal epithelium and stroma. CD157 is expressed by basal limbal clusters, a p63+/cytokeratin 19+ cell subset reported to contain corneal stem cells, and by stromal cells. The results of the work indicates that the human cornea is equipped with molecular tools capable of consuming extracellular NAD+, and that CD157 is a potential marker of corneal limbal cells in the stem cell niche. The presence and characteristics of these ectoenzymes may be exploited to design drugs for wound repair or for applications in tissue transplantation.
INTRODUCTION
The avascular and transparent human cornea is composed of epithelial, stromal, and endothelial layers. The corneal epithelium, which plays vital functional roles both as a physical barrier at the ocular surface and in wound repair and inflammation, must undergo continuous self-renewal through the action of stem cells (1,2). Epithelial stem cells reside in specialized niches at the corneoscleral junction, or limbus (3). A number of signaling pathways have been shown to be involved in the regulation of self-renewing tissues; the pathways involved in corneal epithelium regeneration mostly are unexplored (4).
In many tissues, extracellular NAD+ induces a variety of physiological responses in epithelial cells (5). NAD+ is made available either by passive release from dying cells or by active secretion through connexin 43 (Cx43) channels in a paracrine and in an autocrine way (6). In humans, CD38 and CD157 are the main NAD+-modifying enzymes, synthesizing compounds critical for cell homeostasis and metabolism (7). CD38, a 45-kDa transmembrane glycoprotein, functions both as an ectoenzyme and as a receptor. CD38 is a multifunctional enzyme which converts NAD+ to adenosine diphosphate ribose (ADPR) in neutral pH conditions, through its NADase activity and to cyclic ADPR (cADPR) through its ADP-ribosyl cyclase (ARC) activity. CD38 hydrolyzes cADPR to ADPR through its cADPR hydrolase activity. These end products are active biologically as second messengers: cADPR gates Ca2+ release from intracellular stores, and ADPR can activate the transient receptor potential (TRP) channel-2 leading to Ca2+ influx (8,9). As a receptor, CD38 is involved in the transduction of activation and proliferation signals, thus cooperating in the adhesion of leukocytes to endothelium through binding to its non-substrate ligand CD31 (10). CD38 is expressed in a unique pattern by cells of the immune system, and shows widespread distribution and cellular functions in non-hematopoietic tissues (7).
CD157, a 42- to 45-kDa surface molecule with a glycosylphosphatidylinositol (GPI)-anchor, was identified originally in humans as bone marrow stromal cell-1 (BST-1) antigen (11). CD157 is a second mammalian member of the NADase/ ARC family, and shares 36% amino acid identity and 53% similarity to human CD38. The tissue distribution of CD157 differs significantly from that of CD38 (12); despite their probably different physiological roles, the two glycoproteins nonetheless have similar enzymatic functions. Like CD38, CD157 also is a receptor that transduces activation signals, although a non-substrate ligand has not yet been identified (13).
Increasing evidence indicates that the bioactive molecules cADPR and ADPR are regulators of Ca2+-dependent functions (8). As variations in Ca2+ concentration in ocular compartments also may underlie pathological changes leading to blindness, an investigation into the functional role of the ARC family in corneal tissue is of the utmost importance (14,15). Although reported in the retina in vertebrate eyes (16–18), the expression of NAD+-metabolizing ectoenzymes in the human cornea has been unexplored until now. This work was designed to fill this gap, to add to our body of knowledge concerning the tissue distribution of CD38 and CD157, and to shed light on the possible functional role of ARCs in the health and disease of the human eye.
MATERIALS AND METHODS
Antibodies and Reagents
The anti-CD38 monoclonal antibodies (mAbs) used were IB4 (IgG2a) (19) and AT13/5 (IgG1) (20). The anti-CD157 mAb used was SY/11B5 (IgG1) and the anti-HLA Class I mAb was O1.65 (IgG2a), used as a positive control. All mAbs were produced in-house and purified by HPLC (Beckman Gold 126/166NM) affinity chromatography on Protein A (MAb Select, GE Healthcare, Piscataway, NJ, USA), followed by hydroxyapatite column (BioRad, Milan, Italy), as described (21). Other reagents were polyclonal goat anti-human cytokeratin 3/12 (CK3/12), anti-cytokeratin 19 (CK19), anti-connexin 43 (Cx43), and anti-p63 mAbs, purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). Secondary antibodies for immunofluorescence were: donkey anti-mouse IgG conjugated with Alexa 488 (DαMIgG-Alexa 488) and donkey anti-goat Ig labeled with Alexa 555 (DαGIg-Alexa 555) from Invitrogen (Milan, Italy). All other reagents were of analytical grade and obtained from Sigma (Milan, Italy).
Isolation and Culture of Corneal Cells
Viable epithelial and stromal cells were obtained from human corneoscleral tissues from 10 cadaver eye donors (Piedmont Cornea Bank, San Giovanni Battista Hospital, Torino, Italy) deemed unsuitable for transplantation due to unacceptably low endothelial cell counts or stromal wounds. Epithelial cells were isolated by scraping the ocular surface after detaching the endothelial layer from the stroma, or were cultured from corneal explants. Surface-scraped epithelial cells were counted under a microscope and washed twice in phosphate buffered saline (PBS). Cultured epithelial cells were obtained by outgrowing. Corneas were cut into 12 single sections and placed epithelial side down in 6-well plates (Corning Costar, Milan, Italy) with a drop of Corneal Epithelial Cell EpiLife Medium (Invitrogen), followed 24 h later by an additional 1 mL of medium. After spreading, cells were cultured at 2 × 104 cell/cm2 for 3 to 5 passages. Corneal stromal cells were derived from the en-dothelial and epithelial layers scraped from the donor corneas. The stromal component was released after treatment (3 to 4 h, with gentle shaking) with collagenase IV (2.5 mg/mL in Hank’s balanced salt solution without Ca2+ and Mg2+), and then cultured (5% CO2 , 37°C) in DMEM (all from Gibco, Milan, Italy) supplemented with 2 mM L-glutamine (Invitrogen), 10% FCS (Hyclone, Logan, UT, USA), and 50 IU/mL streptomycin (Sigma).
Immunofluorescence
Surface expression of CD38 and CD157 in corneal cells was determined by indirect immunofluorescence (IIF). Primary cells scraped from the epithelium (2 × 105 cells/sample) or from cultured corneal epithelial and stromal cells were fixed in 2% paraformaldehyde (PAF) for 15 min. Non-specific protein binding was blocked (30 min at 4°C) by incubation with 1% BSA. Cells were washed with PBS and then incubated (1 h at 4°C for surface-scraped cells or 2 h at room temperature for cultured cells) with anti-CD38, anti-CD157, or anti-HLA Class I mAbs. Staining was obtained by incubating the cells (30 min for surface-scraped cells or 90 min for cultured cells at 4°C) with DαMIgG-Alexa 488. The sections were coverslipped in Mounting Medium (Sigma) and viewed under a wide-field or by confocal microscope (Leica TCS SP2, Heidelberg, Germany), using 488 nm and 543 nm laser excitation lines and collected by Leica confocal software. Background fluorescence was determined by means of isotype-matched control mAbs in parallel experiments. Flow cytometry was performed with scraped and cultured epithelial cells analyzed on a FACSort (Becton-Dickinson, San Jose, CA, USA) with CellQuest software.
RNA Extraction and Reverse Transcriptase-PCR (RT-PCR)
Total RNA was extracted from isolated primary corneal cell populations. For each sample, 1.5 × 106 cells were resuspended in TRIzol solution (Invitrogen) and processed according to the manufacturer’s instructions. For cDNA synthesis, 1 to 5 μg of total RNA was reverse transcribed (1 h at 37°C) in the presence of oligo-dT primers. One-fifth of the sample was used per PCR reaction. Full-length transcripts for CD38 and CD157 were amplified using primers that corresponded to their respective 5′ and 3′ UTRs: F38 5′ TCTCTCTTGCTGCCTAGC 3′; R38 5′ AACCACAAGGAGTCAAGG 3′; pBST 5′ GAGATATCCGAGCGAGAG 3′; mBST 5′ AGGACATCGTTTTCCCAG. After 2 min denaturation at 94°C, the 1 kb CD38 transcript was amplified following 40 cycles at 94°C for 30 s, 55°C for 30 s, and 68°C for 1.5 min. The 1.1 kb CD157 amplicon was obtained following 40 cycles at 94°C for 30 s, 60°C for 30 s, and 68°C for 1.5 min. PCR products were analyzed by electrophoresis on a 1% agarose gel. Gels were stained with ethidium bromide and photographed under an ultraviolet (UV) lamp. A negative control lacking the cDNA template was used routinely to rule out contamination.
Immunoprecipitation and Western Blot
Primary isolated epithelial and stromal cells were harvested and lysed as reported (22). Before immunoprecipitation, cell lysates were precleared by incubation with anti-mouse IgG-agarose (Sigma, 1 to 2 h at 4°C) followed by centrifugation (11,000g , 5 s at 4°C). The antibody binding reaction was performed (12 h at 4°C) with anti-CD38 IB4 or anti-CD157 SY/11B5 mAbs (0.2 μg/100 μg of lysate) diluted in PBS containing 1 mg/mL BSA. After incubation (1 to 2 h at 4°C) with anti-mouse IgG-agarose, the reaction mixture was centrifuged (3,000g, 15 min at 4°C). The immunocomplexes then were heat denaturated for 5 min in sample buffer (NuPAGE, Invitrogen). Samples (45 μg/lane) were loaded on a Novex NuPAGE 4% to 12% Bis-Tris precast gel (Invitrogen) and run under non-reducing and reducing conditions. Positive controls (CD38+ Raji B cells and murine NIH-3T3 fibroblasts transfected with the human CD157 gene, NIH-3T3/CD157+), and molecular weight protein markers (Bio-Rad) were run simultaneously with the samples.
For Western blotting, proteins resolved as above were blotted electrophoretically onto PVDF membranes (BioRad) in Tris-glycine buffer. Membranes were stained with 0.2% Ponceau S to assess electrophoretic transfer. The membrane was blocked by 5% BLOTTO (Bio-Rad) in immunoblot buffer (10 mM Tris, pH 7.4, 0.9% NaCl, 0.05% Tween-20, and 1 mM EDTA) for 1 h and then incubated (12 h at 4°C) with primary anti-CD38 (AT13/5, 10 μg/mL) or anti-CD157 (SY/11B5, 5 μg/mL) mAbs in 0.5% BLOTTO. After three washes in immunoblot buffer, goat anti-mouse IgG labeled with horseradish peroxidase (HRP) (Perkin Elmer, Life Science, Milan, Italy) was added (30 min at room temperature). Immunoreactive bands were visualized by ECL (Chemiluminescence Reagent Plus, Perkin Elmer).
Enzymatic Activities: NADase and GDP-Ribosyl Cyclase Assays
The enzymatic activities of intact corneal cells were measured by an HPLC-based assay (23) in terms of degradation of the substrate NAD+ to ADPR (NADase) and by measuring the cyclization of nicotinamide guanine dinucleotide (NGD+), an NAD+ surrogate in the supernatant, to its cell impermeant end-product cGDPR (GDP-ribosyl cyclase). Intact corneal cells (1 × 106 cell/mL) were diluted in a final volume of 1 mL PBS, 5 mM glucose, and either 0.2 mM NAD+ or NGD+. At different incubation times at 37°C, aliquots were withdrawn and supernatants filtered on 0.22 μm-microcentrifuge filters (Sigma). The amount of reaction products was determined on a Beckman Gold NM166 HPLC system, using a reversed-phase C18 column run at room temperature at a flow rate of 0.8 mL/min. Nucleotides were eluted isocratically in 15 mM potassium phosphate buffer, pH 6.7, containing 2.5% (vol/vol) HPLC-grade acetonitrile (Merck, Darm-stadt, Germany) and detected by UV absorbance at 254 nm. The elution times (in min) of standard nucleotides (Sigma) were: NAD+, 6.5; NGD+, 8.0; ADPR, 12.5; and cGDPR, 18. Raji lymphoma (CD38+/ CD157−) and NIH-3T3/CD157+ transfectants were used as positive cell controls.
Immunohistochemical Analysis
Compartmentalization of CD38 and CD157 was determined after IIF tests on frozen corneal cryostat sections. Corneas were included in optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura, Zoeterwoude, the Netherlands), frozen in liquid nitrogen, and stored at −80°C; 7 to 10 μm sections were collected on Superfrost Slides, fixed in 2% PAF for 30 min, and then washed in PBS. When necessary, sections were permeabilized with Triton X-100 (0.3% in PBS) for 5 min. Non-specific protein binding was blocked (1 h at room temperature) by incubation with 1% BSA. Slices then were incubated (12 h at 4°C) with the following mAbs diluted in PBS: anti-CD38 (AT13/5, 10 μg/mL), anti-CD157 (SY/ 11B5, 10 μg/mL), anti-Cx43 (2 μg/mL), anti-CK3/12, anti-CK19 (2 μg/mL), anti-p63 (2 μg/mL), and anti-HLA Class I (10 μg/mL). Control isotype-matched irrelevant mAbs were incubated on parallel slides. After multiple washings, slides were incubated (2 h at room temperature) with DαMIgG-Alexa 488 or DαGIg-Alexa 555 (Invitrogen) as secondary antibodies, mounted, and preserved at −20°C.
RESULTS
Expression of CD38 and CD157 in Human Corneal Cells
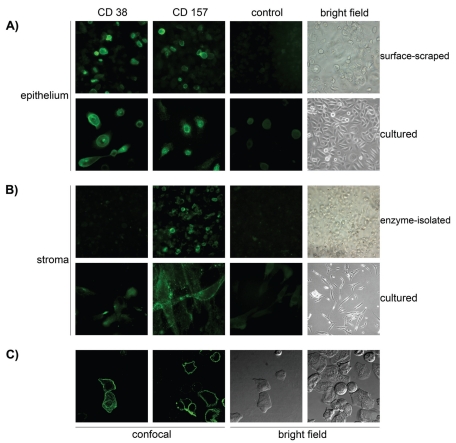
Localization of CD38 and CD157 was examined first by IIF to test whether specific anti-CD38 and anti-CD157 mAbs could stain corneal epithelial and stromal cells. The anti-CD38 IB4 mAb clearly reacted with surface-scraped isolated epithelial cells in all 10 samples examined (Figure 1A). Expression levels varied from CD38+ (n = 6) and CD38high+ (n = 4). No reactivity was detected in cells treated with appropriate isotype-matched mAb controls. Immunolabeling of fixed, dissociated corneal cells observed by confocal microscopy showed that CD38 was located on the surface of corneal epithelial cells (Figure 1C), identified by expression of surface HLA Class I and cytoplasmic CK3/12 (not shown). CD38 was not detected in isolated stromal cells released from the epithelial and endothelial layers after enzymatic treatment (Figure 1B), excluding the possibility that the signal might derive from contaminating stromal cells. These results confirm that CD38 was localized in a discrete subpopulation of human corneal epithelial cells, as reported previously (24). Bearing in mind the discontinuous pattern of expression exhibited by CD38 in lymphoid cells, we analyzed modulation of CD38 expression in corneal epithelial cells maintained in culture. The expression described above in Figure 1A was found to decrease over time from the initial 50% to 60% to ~10% after 7 d in culture (data not shown).
Figure 1.
Immunodetection of the CD38 and CD157 molecules expressed by human corneal cells. Isolated human corneal cells were stained with anti-CD38 (AT13/5, IgG1), anti-CD157 (SY/11B5, IgG1) mAbs, or with an isotype-matched irrelevant control mAb. (A) Staining of CD38 and CD157 with specific mAbs in surface-scraped or cultured epithelial cells, with corresponding control and bright field images. (B) Staining of CD38 and CD157 in enzyme-isolated or cultured stromal cells, with corresponding control and bright field images. (C) Fluorescent images acquired using confocal microscopy, showing the staining of CD38 and CD157 in surface-scraped corneal epithelial cells and corresponding fields seen with interference contrast. The molecules are localized at the membrane levels of the corneal epithelial cells.
Strong CD157 expression was observed by IIF in both primary epithelial and stromal cells (see Figure 1A). Unlike CD38, CD157 expression was maintained over time in cultured cells (see Figure 1B). Confocal microscopy revealed that CD157 expression resembled that of CD38, although in a distinct pattern: CD157 displayed a dot-like pattern of expression of GPI-linked molecules in the plasma cell membranes (see Figure 1A,1C), generally reported in other human cells and tissues (12).
Differential Expression of CD38 and CD157 Transcripts and Proteins in Corneal Epithelium and Stroma
The above results point to differences in pattern of expression of CD38 and CD157 proteins in human corneal tissues. To confirm this, total RNA was isolated from samples of corneal epithelium or stroma: CD38 and CD157 expression was evaluated in each sample by RT-PCR. CD38 transcripts were detected in corneal epithelium, but not in stroma, while CD157 was observed in both corneal layers (Figure 2A). These results confirm the pattern of protein surface expression observed by IIF.
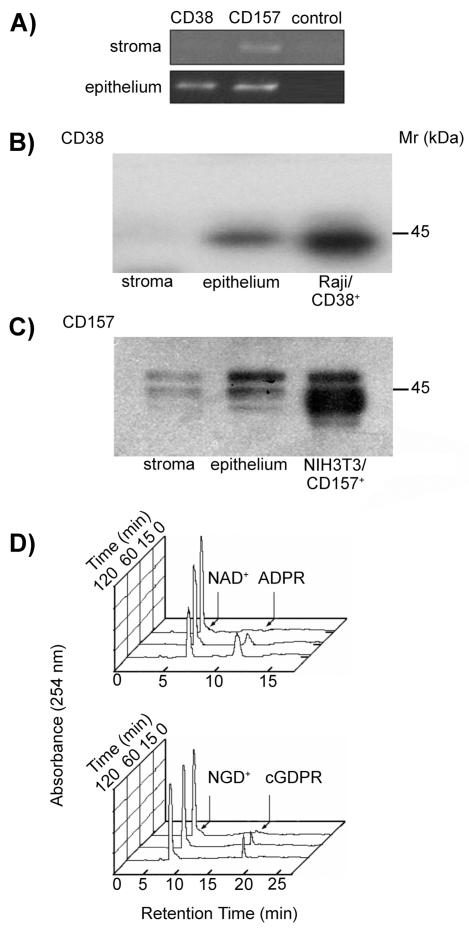
Figure 2.
Biochemical analysis of the CD38 and CD157 molecules expressed by human corneal cells. (A) RT-PCR profile of CD38 and CD157 transcripts in corneal cells. PCR products were amplified from cDNA obtained from epithelial and stromal cells using CD38- or CD157-specific primer sets. Negative controls lacking cDNA template were used to rule out contamination. (B–C) Western blot analysis of corneal stromal and epithelial proteins immunoprecipitated with mAbs to CD38 and CD157. Precipitates of epithelial and stromal cells from biopsied corneas with anti-CD38 (IB4, IgG2a) or with anti-CD157 (SY/11B5, IgG1) mAbs were analyzed by 4% to 12% SDS-PAGE under non-reducing conditions and blotted onto PVDF membranes. Proteins with Mr of 45-kDa were immunodetected in the corneal epithelia and control Raji (CD38+) cells when blots were probed with anti-CD38 (AT13/5, IgG1) mAb and developed by ECL (B). Proteins with Mr of 42- to 45-kDa were immunodetected in the stroma and corneal epithelia when blots were probed with anti-CD157 (SY/11B5, IgG1) mAb and developed by ECL (C). NIH-3T3/CD157+ transfectants were used as positive control. Isotype-matched irrelevant mAbs were used as negative controls. (D) Ectoenzyme activity molecules assessed in epithelial corneal cells. Cells were incubated with 0.2 mM NAD+ or NGD+ at 37°C and samples collected after 0, 15, 60, and 120 min incubations. NADase (upper panel) and GDP-ribosyl cyclase (lower panel) enzymatic activities were determined by HPLC. Raji (CD38+) cells and NIH-3T3/CD157+ transfectants were used as positive controls. K562 (CD38−) cells and NIH-3T3 mock transfectants were the negative controls.
The evidence that the mRNAs detected were translated into surface molecules in intact corneal cells was obtained by Western blotting tests that confirmed the expression and the molecular masses (Mr) of CD38 and CD157. When probed with the IB4 mAb, CD38 featured an ~45-kDa band associated with the plasma cell membranes (Figure 2B). The apparent Mr of human corneal CD38 was indistinguishable from that immunoprecipitated from lymphoid Raji B cells. This finding suggests that a similar degree of glycosylation is maintained in both tissues.
Two bands with a Mr of 42- to 45-kDa were detected when CD157 was precipitated from epithelial and stromal corneal cell lysates probed with the SY/11B5 mAb (Figure 2C). The appearance of CD157 as a two-band molecule was confirmed in NIH-3T3/CD157+ transfectants.
Enzymatic Characterization of Human Corneal CD38 and CD157
After confirming the presence of CD38 and CD157 transcripts and proteins in corneal cells populations, we investigated their enzymatic activities by HPLC-based methods. Incubation of corneal epithelial cells with NAD+ resulted in the progressive disappearance of the nucleotide, eluted at a retention time of 6.5 min (Figure 2D). Concomitant with NAD+ hydrolysis, ADPR eluted 6.0 min after NAD+ (see Figure 2D, upper panel). The NAD+ peak area was correlated with nmoles of NAD+ injected as control, and the specific activity determined from the area under the curve of the NAD+ peak. The NADase activity measured was 0.25 ± 0.04 nmol/ min/106 cells for corneal epithelial cells. NADase activity in control Raji cells (CD38+/CD157−) was measured as 2.51 ± 0.04 nmol/min/106 cells.
The use of NAD+ as substrate may result in an underestimation of ARC activity, due to the subsequent hydrolysis of cADPR (8). Interference of cADPR hydrolase was avoided by using NGD+, a NAD+ surrogate, to assay ARC activity, given that the cGDPR is resistant to hydrolysis (23). This system initially yielded a modest NGD+ cyclization, which was resolved after longer incubations (see Figure 2D, lower panel). ARC activity was 0.05 ± 0.1 nmol/min/106 cells for corneal epithelial cells. Control Raji cells showed marked ARC activity, while K562 (CD38−) cells neither generated cGDPR nor consumed NAD+ (not shown). The ratio between NADase and ARC activities (5:1) for viable intact epithelial corneal cells was comparable with that reported for Raji cells, for CD38 purified from human erythrocytes and for molecules in soluble form in biological fluids (25,26). These results suggest that CD38 and/or CD157 present on the surface of corneal tissues were active enzymatically.
The finding that stromal corneal cells express CD157, but not CD38, prompted us to test their capacity to catalyze the cyclase reaction in the presence of NGD+. Stromal cells (1.0 × 106/sample) yielded low amounts of cGDPR. Thus, stromal cells were intrinsically inefficient at using NGD+ for generating cGDPR at concentrations higher than the threshold sensitivity of the HPLC method adopted. Similar experiments performed with NIH-3T3/CD157+ cells demonstrated that human CD157 could metabolize NGD+. These results are in line with the low catalytic activities reported for CD157 (12).
Topographical Localization of Corneal CD38 and CD157
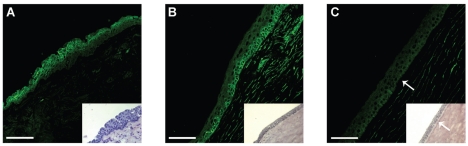
Having demonstrated that functionally active CD38 and CD157 ectoenzymes are expressed constitutively in the cornea, next we turned to fine-mapping the distribution of both proteins by IIF in frozen corneal cryostat sections. The results showed that CD38 was localized in the suprabasal and superficial epithelium layers, limited to the limbal area at the external peripheral structure of the cornea (Figure 3A). CD157 was expressed by the epithelia of the basal limbal zone (Figure 3B), progressively decreasing in the corneal-limbal epithelium (Figure 3C), located above Bowman’s layer.
Figure 3.
Immunostaining of the CD38 and CD157 molecules in cryostat sections of human cornea. Sections were stained with anti-CD38 (AT13/5, IgG1), anti-CD157 (SY/11B5, IgG1) mAbs, and with an isotype-matched irrelevant control mAb. (A) CD38+ cells are visible in the superficial and suprabasal limbal epithelium. No signals are detected in the basal limbal epithelium or in the stroma. (B) CD157+ cells are apparent at the basal limbus and in the stroma. (C) The CD157 signal fade away to the corneal-limbal interface epithelium, above the Bowman’s capsule (arrow). Inset: corresponding bright field images of limbus, corneal-limbal interface, and corneal epithelium (A–C). The junction between limbal and corneal epithelium was determined by the termination of the Bowman’s capsule (arrow). Scale bars: (A) 50 μm; (B,C) 75 μm.
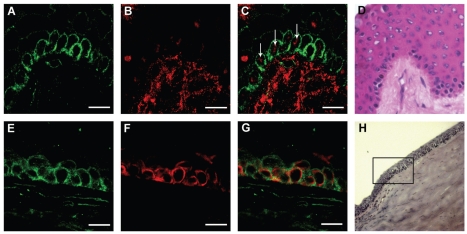
The existence of a discrete CD157+ cell population (see Figure 1C), was confirmed by IIF analysis on fresh normal human corneal tissues (≤ 10 h post mortem). CD157 was expressed by discrete cell clusters, including basal cells at the limbus (Figure 4A–4D). The protein was more apparent in the cells at the center of each cluster, which in turn tended to accumulate at the palisades of Vogt (see Figure 4A), a region of the limbus that harbors the highest concentration of stem cells (1,27). Nuclear p63 antigen is a putative marker of limbal stem cells (28) and, according to merged-double staining, p63+ was coexpressed with CD157 within the basal limbal layer (see Figure 4C).
Figure 4.
Immunostaining of CD157, p63, and CK19 in cryostat sections of human cornea. Sections were stained with anti-CD157 (SY/11B5,IgG1), anti-CK19, and anti-p63 mAbs. Upper images: Staining of CD157 clusters (A) and nuclear p63 (B) in the basal limbal epithelium and the stroma underlying the palisades of Vogt (A–D). Merged confocal image (C) shows that selected CD157+ cells are p63+ (arrows). The corresponding bright field image of the limbus is shown (D). Lower images: Staining of CD157 (E) and CK19 (F) in the basal corneal-limbal epithelium (E–H). The merged confocal image (G) shows that CK19+ cells are CD157+. The corresponding bright field image of the corneal-limbal epithelium is shown (H). Scale bars: 15 μm.
CK19 is expressed by limbal and peripheral corneal epithelial cells (29), suggesting that CD157 and CK19 may express partially overlapped patterns. This hypothesis was addressed by staining serial OCT sections of the limbus and peripheral cornea with anti-CD157 and anti-CK19 mAbs (Figure 4E–4H). CK19 showed an irregular mosaic pattern in the basal limbal epithelial cells (see Figure 4F), in line with a previous report (29). The distribution of CD157 in the basal epithelial cell layer of the limbus (see Figure 4E,4G) resembled that of CK19, which resulted in CK3/12− (not shown), a known marker of corneal epithelial differentiation (30). Moreover, Cx43, a negative marker of human limbal epithelial stem cells (30), was expressed by the corneal epithelium in the suprabasal layer, but could not be detected in the basal epithelial cells of the limbus (not shown). The results from phenotyping human limbal and corneal epithelium with the different mAbs are reported in Table 1.
Table 1.
Surface phenotype of human limbal and corneal epithelia.
| Corneal epithelia
|
Limbal epithelia
|
|||
|---|---|---|---|---|
| Markers | Basal | Suprabasal | Basal | Suprabasala |
| CD38 | −b | − | − | +++c |
| CD157 | − | − | +++ | +/−d |
| p63 | +/− | − | ++e | +f |
| CK19 | − | − | ++ | +/− |
| CK3/12 | ++ | ++ | − | + |
| Cx43 | ++ | + | − | + |
Evaluation based on immunofluorescent staining and empirical score.
−, undetectable.
+++, strongly positive.
+−, staining of the molecule found undetectable (−) or weakly positive (+) in different donors.
++, moderately positive.
+, weakly positive.
DISCUSSION
Our interest in investigating the presence and topology of NAD+-consuming enzymes in human cornea is related to the broader aim of fully characterizing the tissue distribution of CD38 and CD157. Insights from such analysis—in this case, of corneal cells—also will help shed light on functional issues of these ectoenzymes-receptors.
The results obtained in this study indicate that CD38 and CD157 are expressed constitutively by primary corneal cells isolated from human biopsies. CD38 is prevalent on the surface of epithelial cells, while CD157 also is detected on stromal cells, which are CD38−. The simultaneous expression of CD38 and CD157 in the corneal epithelial cells was an unexpected finding, since the two molecules generally are considered mutually exclusive in terms of expression outside the immune system (7). Notwithstanding that, the presence of CD38 and CD157 was confirmed at mRNA and protein levels, ruling out potential mAb cross reactivities. Further, the use of specific mAbs allowed us to explore the biochemical and functional characteristics of the two molecules in human corneal cells. Corneal tissue CD38 is a monomer of ~45-kDa, a main difference from the same molecule expressed by leukocytes, where it is reported as an oligomeric complex. The monomeric form likely is compatible with corneal functions. Indeed, oligomeric CD38 is formed prevalently by transglutamination, a process not fitting the physical properties (viz., ocular transparency) of normal corneal tissues (32,33). The oligomeric asset generally is reported in hematopoietic cells, where it plays a role in stabilizing the molecule on the plasma membrane (34).
Corneal CD157 appears as a doublet with a Mr of 42- to 45-kDa, suggesting the existence of different isoforms, likely produced by alternative glycosylation (35). Precipitates from stromal corneal cells and NIH-3T3/CD157+ transfectants blotted under non-reducing conditions display an additional band of ~150-kDa. The same test performed in reducing conditions revealed only monomeric 42-to 45-kDa chains, indicating that intermolecular disulfide bond(s) account for the higher Mr complexes. It may be relevant to investigate whether the oligomers are composed of homo- or of hetero-oligomers of CD157, covalently bound with a yet unidentified protein.
The joint expression of CD38 and CD157 in a population of human corneal cells might represent an evolutionary strategy providing survival mechanisms in critical situations. At the same time, this finding adds clues as to the functional roles played by the molecules. Both molecules expose their enzymatic sites to the extracellular milieu in conditions ideal for reaction with NAD+, which is extruded efficiently across the plasma membrane of corneal epithelial cells through Cx43 channels (6,31). One would guess that cell death, inflammation, and corneal wound healing represent different sources of NAD+ in this environment. The results obtained in this work indicate that intact corneal cells express the enzymatic activities attributed to the extracellular domain of native CD38. Indeed, cells consume extracellular NAD+ and form cADPR, even if in low amounts. The cyclase activity was evaluated by measuring the ability of CD38 to cyclize NGD+. In these conditions, the NADase/ARC activity ratio of intact corneal cells is similar to that measured in hematopoietic cell preparations (26). Stromal cells (CD157+) display very low enzymatic activity, as observed in other cell lineages (35,36). These results demonstrate that the corneal epithelial tissue is characterized by molecules featuring NAD+ consumption. CD38 and CD157 are the only human enzymes known to feature NADases/ARC activities (8). Consequently, CD38 is most likely responsible for nearly all the enzymatic activities observed in corneal epithelial cells. According to these observations, one could envisage a model in which CD38 on the surface of corneal epithelial cells first senses an increase of extracellular NAD+ and then responds by scavenging the extracellular nucleotide.
Recent reports tie in with the model inferred from the results of this study: indeed, retinal vasotoxicity can be triggered by extracellular NAD+ soon after the onset of diabetic retinopathy, characterized by reduced scavenging by microvessels of the extracellular nucleotide (37,38). In line with these observations, one would speculate that the generation of anti-CD38 autoantibodies block NAD+ consumption by CD38 in diabetic patients (39) and consequently threaten eyesight. This hypothesis is supported indirectly by the transient blindness effects observed during the first in vivo test with anti-CD38 mAbs, used for experimental treatment of human hematological malignancies (TJ Hamblin, Southampton, UK, personal communication). This issue is timely now that the first human anti-human CD38 mAbs are available for clinical trials (40).
Epithelium regenerates during wound repair by migration of cells from the corneal limbus (1,28). However, an open issue in corneal regeneration is the lack of a dependable surface marker of the limbal stem cell population (41). The results of this work show that CD38 and CD157 mark subsets of human corneal epithelial cells. The corneal epithelium does not express CD38, on the contrary, detectable on the suprabasal and superficial limbal epithelium. CD157 is expressed by the basal epithelial layer of the limbus, where it appears in clusters of highly stained cells coexpressing nuclear p63, a molecule apparently required for the migration of limbal cells (28,42). Recent studies support the hypothesis that CD157 is one of a coordinated series of events linked to hematopoietic cell migration (7). The inference that could be extrapolated from the present results is that CD157 may be a useful marker for the identification and isolation of human limbal epithelial cells that share characteristics attributed to corneal stem cells. We thus conclude that the basal epithelial cells of the human limbus are of CD157+, CD38−, p63+, CK19+, CK3/12−, and Cx43−phenotype. Therefore, a peculiar combination of markers of corneal epithelial differentiation (for example, CK3/12, Cx43, and CD38) and limbal cell-associated markers (for example, p63, CK19, and CD157) may allow identification of human corneal stem cells.
These results show that the normal human cornea is equipped with a molecular tool to actively metabolize NAD+, helping maintain corneal homeostasis. The presence of these ectoenzymes may pave the way to the design of novel drugs to control wound repair. Lastly, the role of CD38 and CD157 as corneal surface receptors is currently being examined to obtain sound experimental evidence to translate biological information back to a clinical set, such as tissue transplantation.
ACKNOWLEDGMENTS
Work supported by grants from Telethon (ALH) and by the special project “Oncologia” Compagnia SanPaolo (Torino, Italy). FIRMS (Fondazione Internazionale Ricerca in Medicina Sperimentale) provided valuable financial contributions. ALH and RL were supported by FIRMS Research Fellowships.
We thank George Stevenson (Tenovus Research Laboratory, Southampton University Hospital, UK) for providing the AT13/5 anti-CD38 mAb. We also thank G Mazzucco (Electronic Microscopy Lab, Department of Biomedical Science, Torino) for advice with corneal cryostat sectioning.
Footnotes
DISCLOSURE
We declare that the authors have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 2.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–46. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg K, Brown ME, Chaves HV, Kenyon KR, Rheinwald JG. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34:2672–9. [PubMed] [Google Scholar]
- 4.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 5.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 6.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. Faseb J. 2001;15:10–2. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 7.Malavasi F, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12:317–323. doi: 10.2119/2006-00086.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol. 2006;17:654–66. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Deaglio S, et al. Human CD38 ligand. A 120-kDa protein predominantly expressed on endothelial cells. J Immunol. 1996;156:727–34. [PubMed] [Google Scholar]
- 11.Kaisho T, et al. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc Natl Acad Sci U S A. 1994;91:5325–9. doi: 10.1073/pnas.91.12.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortolan E, et al. CD157, the Janus of CD38 but with a unique personality. Cell Biochem Funct. 2002;20:309–22. doi: 10.1002/cbf.978. [DOI] [PubMed] [Google Scholar]
- 13.Malavasi F, et al. CD38 and CD157 as receptors of the immune system: a bridge between innate and adaptive immunity. Mol Med. 2006;12:334–41. doi: 10.2119/2006-00094.Malavasi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan G, Williams MR, Riach RA. Calcium, cell signalling and cataract. Prog Retin Eye Res. 1994;13:623–52. [Google Scholar]
- 15.Chattopadhyay N, et al. Expression of extracellular calcium-sensing receptor by human lens epithelial cells. Biochem Biophys Res Commun. 1997;233:801–5. doi: 10.1006/bbrc.1997.6553. [DOI] [PubMed] [Google Scholar]
- 16.Khoo KM, Chang CF. Characterization and localization of CD38 in the vertebrate eye. Brain Res. 1999;821:17–25. doi: 10.1016/s0006-8993(98)01347-x. [DOI] [PubMed] [Google Scholar]
- 17.Esguerra M, Miller RF. CD38 expression and NAD+-induced intracellular Ca+ mobilization in isolated retinal Muller cells. Glia. 2002;39:314–9. doi: 10.1002/glia.10115. [DOI] [PubMed] [Google Scholar]
- 18.Panfoli I, et al. Localization of the cyclic ADP-ribose-dependent calcium signaling pathway in bovine rod outer segments. Invest Ophthalmol Vis Sci. 2007;48:978–84. doi: 10.1167/iovs.06-0543. [DOI] [PubMed] [Google Scholar]
- 19.Malavasi F, et al. Characterization of a murine monoclonal antibody specific for human early lymphohemopoietic cells. Hum Immunol. 1984;9:9–20. doi: 10.1016/0198-8859(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 20.Ausiello CM, et al. Functional topography of discrete domains of human CD38. Tissue Antigens. 2000;56:539–47. doi: 10.1034/j.1399-0039.2000.560608.x. [DOI] [PubMed] [Google Scholar]
- 21.Horenstein AL, Durelli I, Malavasi F. Purification of clinical-grade monoclonal antibodies by chromatographic methods. Methods Mol Biol. 2005;308:191–208. doi: 10.1385/1-59259-922-2:191. [DOI] [PubMed] [Google Scholar]
- 22.Horenstein AL, Stockinger H, Imhof BA, Malavasi F. CD38 binding to human myeloid cells is mediated by mouse and human CD31. Biochem J. 1998;330:1129–35. doi: 10.1042/bj3301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graeff RM, Walseth TF, Fryxell K, Branton WD, Lee HC. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J Biol Chem. 1994;269:30260–7. [PubMed] [Google Scholar]
- 24.Sizzano F, et al. Identification of the ectoenzyme-receptor CD38 on human corneal epithelial cells [abstract] Tissue Antigens. 2007;390;69 21st European Immunogenetics and Histocompatibility Conference; 2007 May 5–8; Barcelona, Spain. [Google Scholar]
- 25.Zocchi E, et al. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem. Biophys. Res. Commun. 1993;196:1459–65. doi: 10.1006/bbrc.1993.2416. [DOI] [PubMed] [Google Scholar]
- 26.Funaro A, et al. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int Immunol. 1996;8:1643–50. doi: 10.1093/intimm/8.11.1643. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–66. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Iorio E, et al. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A. 2005;102:9523–8. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauweryns B, van den Oord JJ, De Vos R, Missotten L. A new epithelial cell type in the human cornea. Invest Ophthalmol Vis Sci. 1993;34:1983–90. [PubMed] [Google Scholar]
- 30.Harkin DG, Barnard Z, Gillies P, Ainscough SL, Apel AJ. Analysis of p63 and cytokeratin expression in a cultivated limbal autograft used in the treatment of limbal stem cell deficiency. Br J Ophthalmol. 2004;88:1154–8. doi: 10.1136/bjo.2003.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shurman DL, Glazewski L, Gumpert A, Zieske JD, Richard G. In vivo and in vitro expression of connexins in the human corneal epithelium. Invest Ophthalmol Vis Sci. 2005;46:1957–65. doi: 10.1167/iovs.04-1364. [DOI] [PubMed] [Google Scholar]
- 32.Umar S, Malavasi F, Mehta K. Post-translational modification of CD38 protein into a high molecular weight form alters its catalytic properties. J Biol Chem. 1996;271:15922–7. doi: 10.1074/jbc.271.27.15922. [DOI] [PubMed] [Google Scholar]
- 33.Tong L, et al. Transglutaminase participates in UVB-induced cell death pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:4295–301. doi: 10.1167/iovs.06-0412. [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Garcia ME, et al. CD38 is expressed as noncovalently associated homodimers on the surface of murine B lymphocytes. Eur J Biochem. 2004;271:1025–34. doi: 10.1111/j.1432-1033.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirata Y, et al. ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett. 1994;356:244–8. doi: 10.1016/0014-5793(94)01279-2. [DOI] [PubMed] [Google Scholar]
- 36.Hussain AM, Lee HC, Chang CF. Functional expression of secreted mouse BST-1 in yeast. Protein Expr Purif. 1998;12:133–7. doi: 10.1006/prep.1997.0811. [DOI] [PubMed] [Google Scholar]
- 37.Liao SD, Puro DG. NAD+-induced vaso-toxicity in the pericyte-containing microvasculature of the rat retina: effect of diabetes. Invest Ophthalmol Vis Sci. 2006;47:5032–8. doi: 10.1167/iovs.06-0422. [DOI] [PubMed] [Google Scholar]
- 38.Dianzani U, et al. Interaction between endothelium and CD4+CD45RA+ lymphocytes. Role of the human CD38 molecule. J Immunol. 1994;153:952–9. [PubMed] [Google Scholar]
- 39.Antonelli A, et al. Human anti-CD38 autoantibodies raise intracellular calcium and stimulate insulin release in human pancreatic islets. Diabetes. 2001;50:985–91. doi: 10.2337/diabetes.50.5.985. [DOI] [PubMed] [Google Scholar]
- 40.Tesar M. Fully human antibody MOR202 against CD38 for the treatment of multiple myeloma and other blood-borne malignancies [abstract] J. Clin. Oncol. 2007;25:8106. (abstract no.). 2007 ASCO Annual Meeting proceedings (post-meeting edition) of the American Society of Clinical Oncology. [Google Scholar]
- 41.Chee KY, Kicic A, Wiffen SJ. Limbal stem cells: the search for a marker. Clin Experiment Ophthalmol. 2006;34:64–73. doi: 10.1111/j.1442-9071.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 42.Pellegrini G, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–82. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]