Abstract
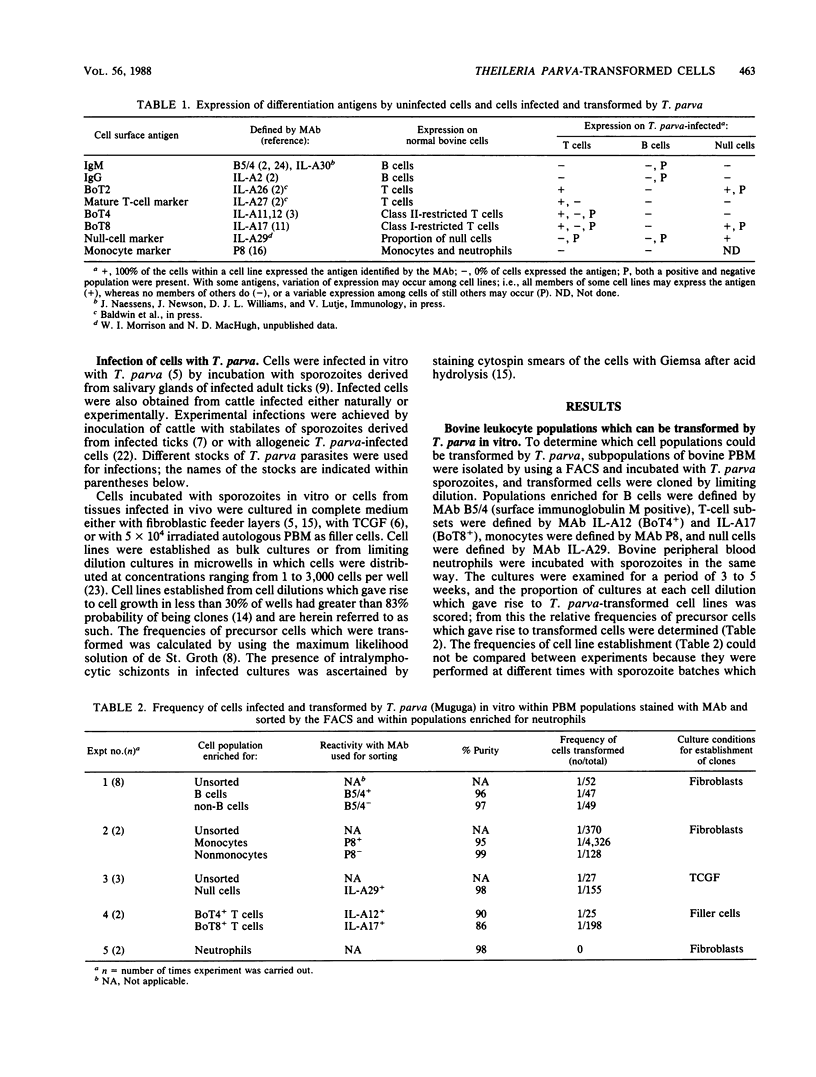
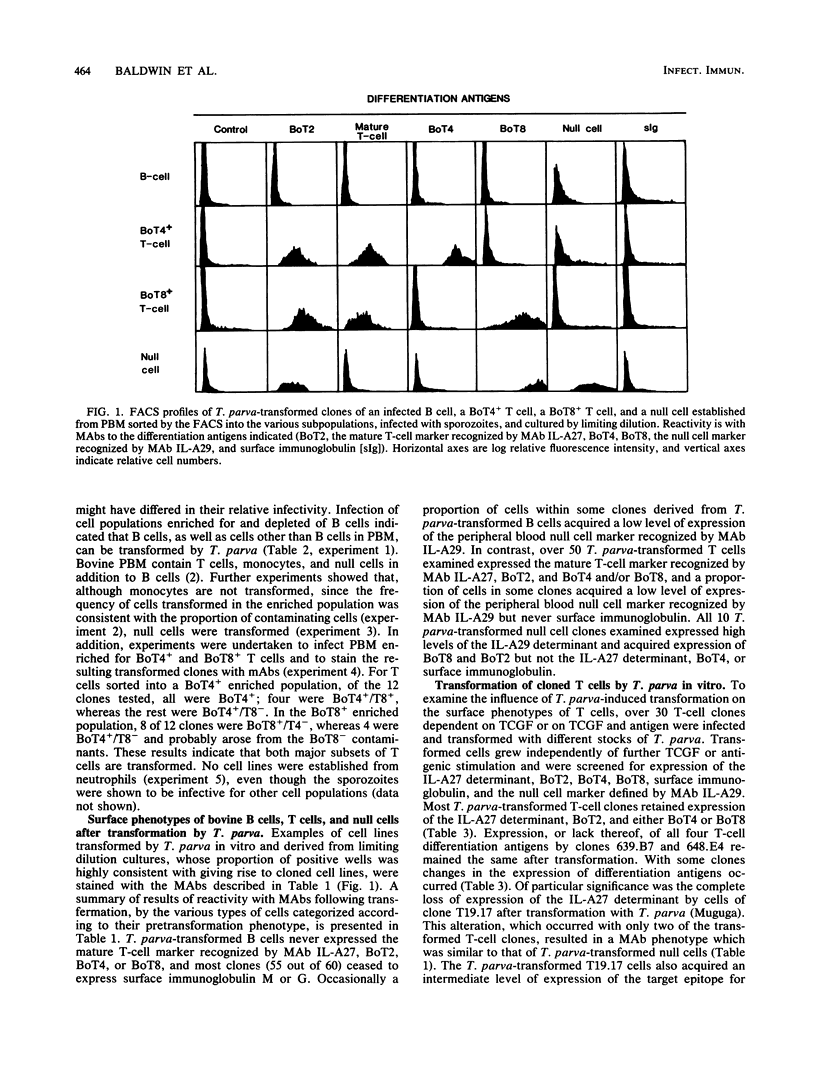
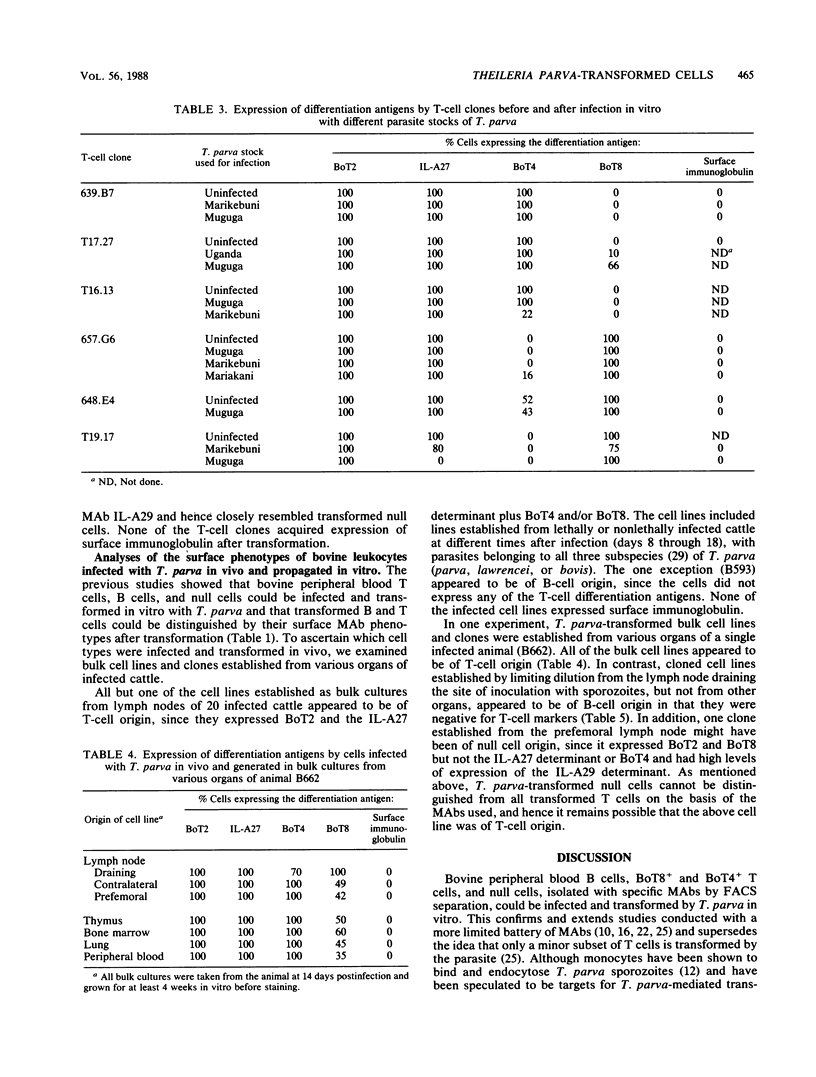
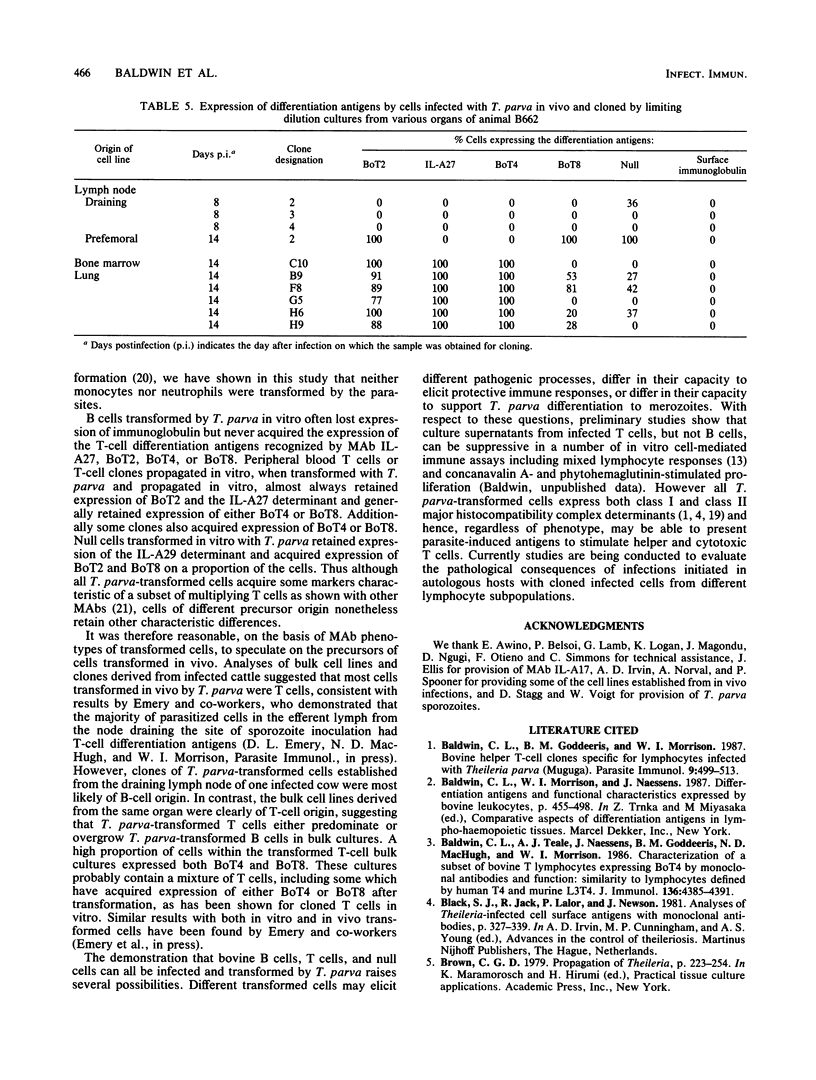
The target cells for infection and transformation by Theileria parva were investigated. Peripheral blood mononuclear cells were reacted with monoclonal antibodies specific for bovine leukocyte differentiation antigens, sorted into subpopulations with a fluorescence-activated cell sorter, and infected in vitro with T. parva sporozoites. Infected cells were cultured at limiting dilution, and transformed clones were screened with monoclonal antibodies. The results indicated that B cells, T cells (including BoT4+ and BoT8+ cells), and null cells but not monocytes or neutrophils were transformed in vitro after infection with T. parva. After transformation, peripheral blood T cells and T-cell clones retained expression of most or all of the T-cell differentiation antigens including the mature T-cell marker recognized by monoclonal antibody IL-A27, BoT2, and BoT4 or BoT8, and some cells acquired a low level of expression of BoT4, BoT8, or the null cell marker recognized by monoclonal antibody IL-A29. T. parva-transformed null cells retained expression of the IL-A29 determinant and acquired expression of BoT2 and BoT8 but not the IL-A27 determinant or BoT4. T. parva-transformed B cells in most instances lost expression of surface immunoglobulin and never acquired expression of the IL-A27 determinant, BoT2, BoT4, or BoT8, although some cells acquired a low level of expression of the null cell marker recognized by monoclonal antibody IL-A29. Further studies on cell lines and clones grown in vitro from populations isolated from T. parva-infected cattle suggested that the majority of the in vivo T. parva-transformed cells were of T-cell origin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Goddeeris B. M., Morrison W. I. Bovine helper T-cell clones specific for lymphocytes infected with Theileria parva (Muguga). Parasite Immunol. 1987 Jul;9(4):499–513. doi: 10.1111/j.1365-3024.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Baldwin C. L., Teale A. J., Naessens J. G., Goddeeris B. M., MacHugh N. D., Morrison W. I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986 Jun 15;136(12):4385–4391. [PubMed] [Google Scholar]
- Brown W. C., Logan K. S. Bovine T-cell clones infected with Theileria parva produce a factor with IL 2-like activity. Parasite Immunol. 1986 Mar;8(2):189–192. doi: 10.1111/j.1365-3024.1986.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Cunningham M. P., Brown C. G., Burridge M. J., Irvin A. D., Purnell R. E., Radley D. E. Theileria parva: comparative infectivity of a ground tick stabilate and a classical 10-tick challenge. Res Vet Sci. 1973 Sep;15(2):263–265. [PubMed] [Google Scholar]
- Dobbelaere D. A., Spooner P. R., Barry W. C., Irvin A. D. Monoclonal antibody neutralizes the sporozoite stage of different Theileria parva stocks. Parasite Immunol. 1984 Jul;6(4):361–370. doi: 10.1111/j.1365-3024.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Duffus W. P., Wagner G. G., Preston J. M. Initial studies on the properties of a bovine lymphoid cell culture line infected with Theileria parva. Clin Exp Immunol. 1978 Dec;34(3):347–353. [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., Stagg D. A. Passive endocytosis of sporozoites of Theileria parva in macrophages at 1-2 degrees C. J Submicrosc Cytol. 1986 Jan;18(1):11–19. [PubMed] [Google Scholar]
- Fazekas de St Groth The evaluation of limiting dilution assays. J Immunol Methods. 1982 Mar 12;49(2):R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- Goddeeris B. M., Morrison W. I. The bovine autologous Theileria mixed leucocyte reaction: influence of monocytes and phenotype of the parasitized stimulator cell on proliferation and parasite specificity. Immunology. 1987 Jan;60(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Kurtti T. J., Munderloh U. G., Irvin A. D., Büscher G. Theileria parva: early events in the development of bovine lymphoblastoid cell lines persistently infected with macroschizonts. Exp Parasitol. 1981 Oct;52(2):280–290. doi: 10.1016/0014-4894(81)90083-7. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Morrison W. I., Goddeeris B. M., Jack R. M., Black S. J. Monoclonal antibodies identify phenotypically and functionally distinct cell types in the bovine lymphoid system. Vet Immunol Immunopathol. 1986 Sep;13(1-2):121–140. doi: 10.1016/0165-2427(86)90054-1. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Buscher G., Murray M., Emery D. L., Masake R. A., Cook R. H., Wells P. W. Theileria parva: kinetics of infection in the lymphoid system of cattle. Exp Parasitol. 1981 Oct;52(2):248–260. doi: 10.1016/0014-4894(81)90080-1. [DOI] [PubMed] [Google Scholar]
- Moulton J., Buscher G., Bovell D., Doxsey S. Blast transformation of adherent macrophages infected in vitro with sporozoites of Theileria parva. Am J Vet Res. 1984 Apr;45(4):678–684. [PubMed] [Google Scholar]
- Naessens J., Newson J., Bensaid A., Teale A. J., Magondu J. G., Black S. J. De novo expression of T cell markers on Theileria parva-transformed lymphoblasts in cattle. J Immunol. 1985 Dec;135(6):4183–4188. [PubMed] [Google Scholar]
- Newson J., Naessens J., Stagg D. A., Black S. J. A cell surface antigen associated with Theileria parva lawrencei-infected bovine lymphoid cells. Parasite Immunol. 1986 Mar;8(2):149–158. doi: 10.1111/j.1365-3024.1986.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Pinder M., Musoke A. J., Morrison W. I., Roelants G. E. The bovine lymphoid system. III. A monoclonal antibody specific for bovine cell surface and serum IgM. Immunology. 1980 Jul;40(3):359–365. [PMC free article] [PubMed] [Google Scholar]
- Pinder M., Withey K. S., Roelants G. E. Theileria parva parasites transform a subpopulation of T lymphocytes. J Immunol. 1981 Jul;127(1):389–390. [PubMed] [Google Scholar]
- Teale A. J., Baldwin C. L., Ellis J. A., Newson J., Goddeeris B. M., Morrison W. I. Alloreactive bovine T lymphocyte clones: an analysis of function, phenotype, and specificity. J Immunol. 1986 Jun 15;136(12):4392–4398. [PubMed] [Google Scholar]


