Abstract
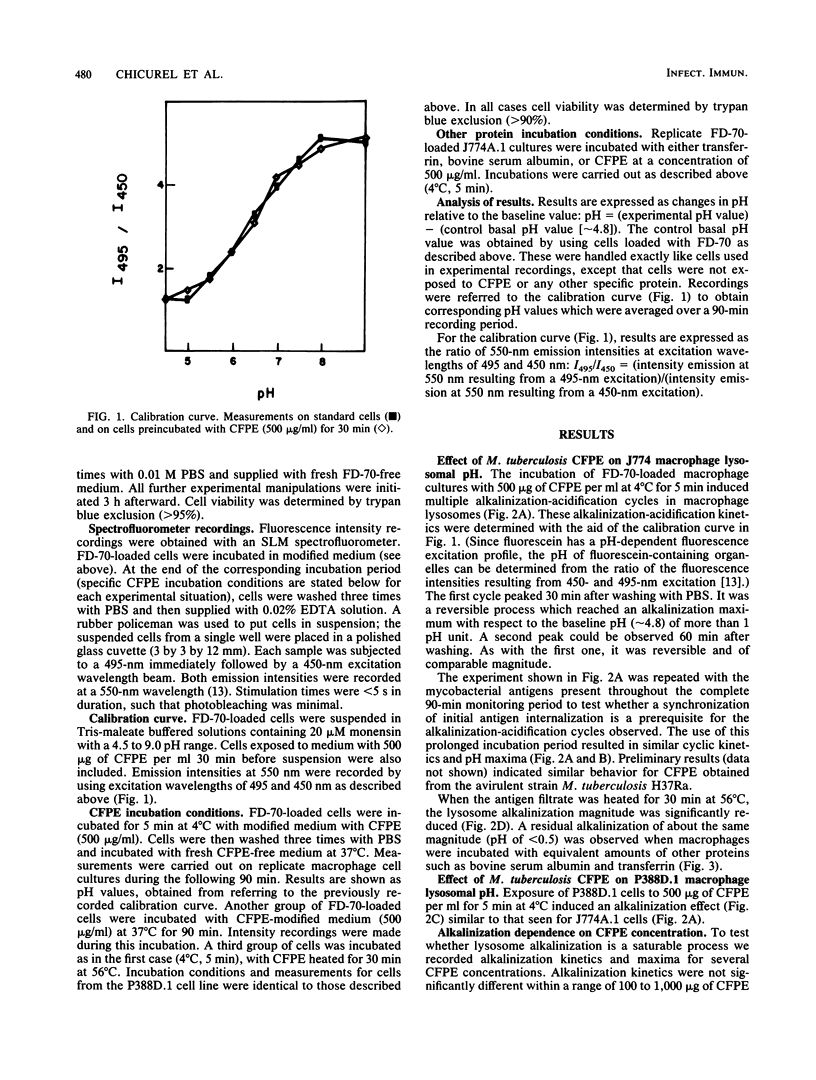
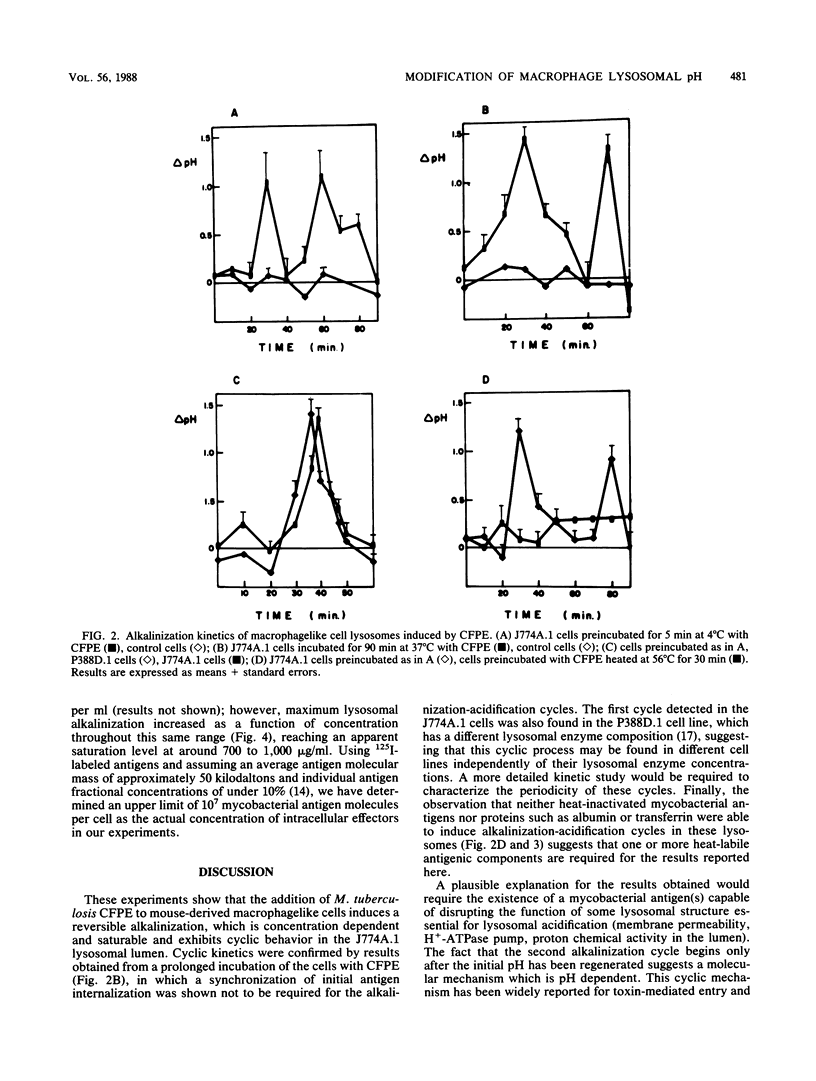
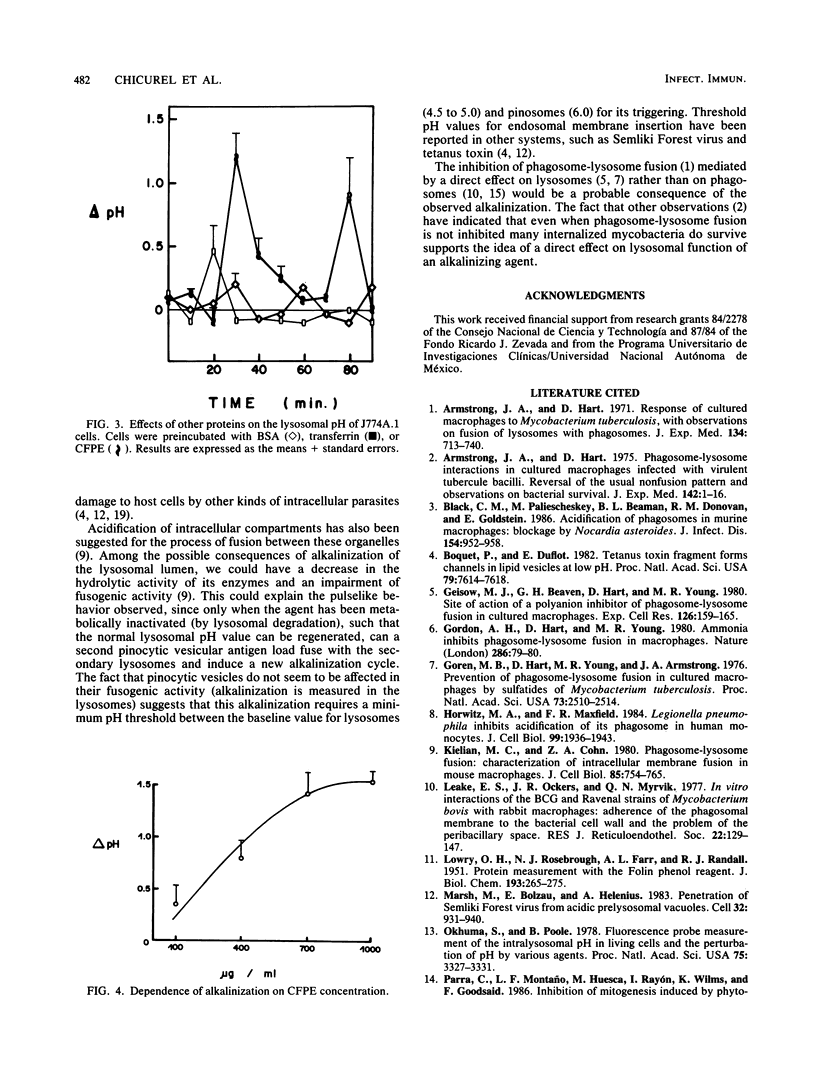
Macrophage lysosomal pH was significantly (greater than 1 pH unit) increased in a reversible, concentration-dependent manner characterized by a saturable and cyclic kinetics after exposure to culture filtrate protein extract derived from Mycobacterium tuberculosis. Lysosomal alkalinization peaked 30 min after administration of culture filtrate protein extract to cells of the macrophagelike cell line J774A.1. The alkalinization was reversible, and a second peak was observed approximately 60 min after incubation. Maximum lysosomal alkalinization increased as a function of culture filtrate protein extract concentration, reaching an apparent saturation level around 700 to 1,000 micrograms/ml, although the time course for this process was not significantly dependent on antigen concentration. The alkalinizing agent(s) was heat labile and produced a similar effect in cells which had a different lysosomal enzyme composition. Our observations are consistent with the presence of one or more mycobacterial antigens which have a pH-dependent affinity for lysosomal structures essential for lysosomal acidification and which are able to inhibit this lysosomal acidification.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Paliescheskey M., Beaman B. L., Donovan R. M., Goldstein E. Acidification of phagosomes in murine macrophages: blockage by Nocardia asteroides. J Infect Dis. 1986 Dec;154(6):952–958. doi: 10.1093/infdis/154.6.952. [DOI] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., Beaven G. H., Hart P. D., Young M. R. Site of action of a polyanion inhibitor of phagosome-lysosome fusion in cultured macrophages. Exp Cell Res. 1980 Mar;126(1):159–165. doi: 10.1016/0014-4827(80)90481-4. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980 Jun;85(3):754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leake E. S., Ockers J. R., Myrvik Q. N. In vitro interactions of the BCG and Ravenel strains of Mycobacterium bovis with rabbit macrophages: adherence of the phagosomal membrane to the bacterial cell wall and the problem of the peribacillary space. J Reticuloendothel Soc. 1977 Aug;22(2):129–147. [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Mizuno D. Different arrangements of phagolysosome membranes which depend upon the particles phagocytized. Observation with markers of the two sides of plasma membranes. Exp Cell Res. 1978 Feb;111(2):437–449. doi: 10.1016/0014-4827(78)90188-x. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Weidner E., Krahenbuhl J. L. Phagosome acidification blocked by intracellular Toxoplasma gondii. 1985 May 30-Jun 5Nature. 315(6018):416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Goldstein I., Hoffstein S. Prostaglandins and the modulation by cyclic nucleotides of lysosomal enzyme release. Adv Prostaglandin Thromboxane Res. 1976;2:803–814. [PubMed] [Google Scholar]
- Yoshimura A., Ohnishi S. Uncoating of influenza virus in endosomes. J Virol. 1984 Aug;51(2):497–504. doi: 10.1128/jvi.51.2.497-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., van Schadewijk-Nieuwstad M., Elzenga-Claasen I., Cornelisse C., Nibbering P. Morphological, cytochemical, functional, and proliferative characteristics of four murine macrophage-like cell lines. Cell Immunol. 1985 Feb;90(2):339–357. doi: 10.1016/0008-8749(85)90199-6. [DOI] [PubMed] [Google Scholar]


