Abstract
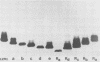
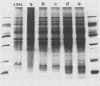
The identification of enterobacterial mutants that contain alterations in the lipopolysaccharide (LPS) oligosaccharide core structure facilitated the development of the model of the physicochemical and immunochemical structures of enteric LPS. Results of recent immunochemical studies have suggested that the structural model of the lipooligosaccharides (LOSs) of Neisseria gonorrhoeae may differ from the enteric LPS model. The difficulties in the analysis of the wild-type gonococcal LOS have precluded understanding of the precise nature of the LOS structure. This study was undertaken to isolate a series of mutants of N. gonorrhoeae 1291 that had sequential saccharide deletions in the LOS. Results of preliminary studies suggested that the pyocin, designated pyocin C, allowed selection of gonococci with such mutant LOS structures. Results also indicated that the receptor for pyocin C binding was an LOS component. Pyocin C selection led to the isolation of five strains with LOS patterns on sodium dodecyl sulfate-polyacrylamide gels which differed from the LOS of parent strain 1291. In this system, the Mr of the parent LOS was 4,715, while the LOSs from the mutant strains demonstrated progressive saccharide deletions, with Mrs of 4,230, 4,089, 3,627, 3,262, and 3,197. Protein patterns of these mutants on sodium dodecyl sulfate-polyacrylamide gels were qualitatively similar to those of the parent strains. Results of studies with five monoclonal antibodies specific for neisserial LOS indicated that shared as well as unique epitopes were present on the mutant LOSs. Results of ketodeoxyoctonate analysis of the mutant LOSs indicated that the majority of the ketodeoxyoctonate residues may be substituted on C-4 or C-5. Chemical and immunological analysis of such LOS mutants should expedite the development of the model for the structure of gonococcal LOS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J Infect Dis. 1974 Dec;130(6):619–625. doi: 10.1093/infdis/130.6.619. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Westerink M. A., Morse S. A., Schneider H., Rice P. A., Griffiss J. M. Bactericidal antibody response of normal human serum to the lipooligosaccharide of Neisseria gonorrhoeae. J Infect Dis. 1986 Mar;153(3):520–526. doi: 10.1093/infdis/153.3.520. [DOI] [PubMed] [Google Scholar]
- Blackwell C. C., Law J. A. Typing of non-serogroupable Neisseria meningitidis by means of sensitivity to R-type pyocines of Pseudomonas aeruginosa. J Infect. 1981 Dec;3(4):370–378. doi: 10.1016/s0163-4453(81)91996-4. [DOI] [PubMed] [Google Scholar]
- Connelly M. C., Stein D. C., Young F. E., Morse S. A., Allen P. Z. Interaction with lectins and differential wheat germ agglutinin binding of pyocin 103-sensitive and -resistant Neisseria gonorrhoeae. J Bacteriol. 1981 Dec;148(3):796–803. doi: 10.1128/jb.148.3.796-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Esser M., Shafer W. M. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect Immun. 1982 May;36(2):541–547. doi: 10.1128/iai.36.2.541-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Westphal O. The significance of enterobacterial mutants for the chemical investigation of their cell-wall polysaccharides. Angew Chem Int Ed Engl. 1966 Feb;5(2):198–210. doi: 10.1002/anie.196601981. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Vaughan P., Johnson D., Iglewski B. H. Inhibition of Neisseria gonorrhoeae by a bacteriocin from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Aug;10(2):354–362. doi: 10.1128/aac.10.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLOSSHARDT J. UNTERSUCHUNGEN UEBER DIE ENTSTEHUNG VON MUTAGENEN IM ZELLSTOFFWECHSEL UND IHRE ROLLE IM S-R-FORMENWECHSEL BEI SALMONELLEN. Zentralbl Bakteriol Orig. 1964 Feb;192:54–66. [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V., Hitchcock P. J. A spontaneous mutant of Neisseria gonorrhoeae with decreased resistance to neutrophil granule proteins. J Infect Dis. 1986 May;153(5):910–917. doi: 10.1093/infdis/153.5.910. [DOI] [PubMed] [Google Scholar]
- Sidberry H. D., Sadoff J. C. Pyocin sensitivity of Neisseria gonorrhoeae and its feasibility as an epidemiological tool. Infect Immun. 1977 Feb;15(2):628–637. doi: 10.1128/iai.15.2.628-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Clark V. L., Young F. E. Inhibition of active transport and macromolecular synthesis by pyocin 103 in Neisseria gonorrhoeae. Sex Transm Dis. 1983 Jan-Mar;10(1):7–13. doi: 10.1097/00007435-198301000-00002. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Winstanley F. P., Blackwell C. C., Tan E. L., Patel P. V., Parsons N. J., Martin P. M., Smith H. Alteration of pyocin-sensitivity pattern of Neisseria gonorrhoeae is associated with induced resistance to killing by human serum. J Gen Microbiol. 1984 May;130(5):1303–1306. doi: 10.1099/00221287-130-5-1303. [DOI] [PubMed] [Google Scholar]