Abstract
Hamster trachea epithelial (HTE) cells were employed as a model system for Mycoplasma pneumoniae pathogenesis. To more closely mimic natural infection, M. pneumoniae was forced to rely upon host cells (as opposed to the growth medium) for nutrients, and infections were initiated with relatively low mycoplasma doses and monitored for extended time periods. A time- and dose-dependent decline in the viability of infected cells was observed; however, viability never declined below 50% of that in uninfected controls. Protein and RNA synthesis actually increased above control levels in infected cells, despite a concomitant decrease in viability. This response was pronounced at higher multiplicities of infection but was only transient at lower doses. In parallel studies in which a culture medium capable of supporting M. pneumoniae growth was used, loss of viability was accelerated. With a low-dose infection a transient increase followed by a precipitous decline in macromolecular synthesis was observed, relative to that in uninfected controls. At higher doses, however, macromolecular synthesis decreased dramatically and in proportion to the loss of viability. The requirement for HTE cells for mycoplasma growth under the experimental culture conditions was demonstrated by quantitating viable mycoplasmas in the culture medium in the presence or absence of HTE cells over 4 days. The increase in mycoplasma number was negligible in the absence of HTE cells, while a 30-fold increase was observed in the presence of HTE cells. These findings demonstrate the feasibility of long-term, low-dose studies of M. pneumoniae pathogenesis with trachea epithelial cells and a nonpermissive culture medium. This experimental system should facilitate the elucidation of the mechanism(s) responsible for host cell injury, and perhaps reveal how host cells respond to infection.
Full text
PDF

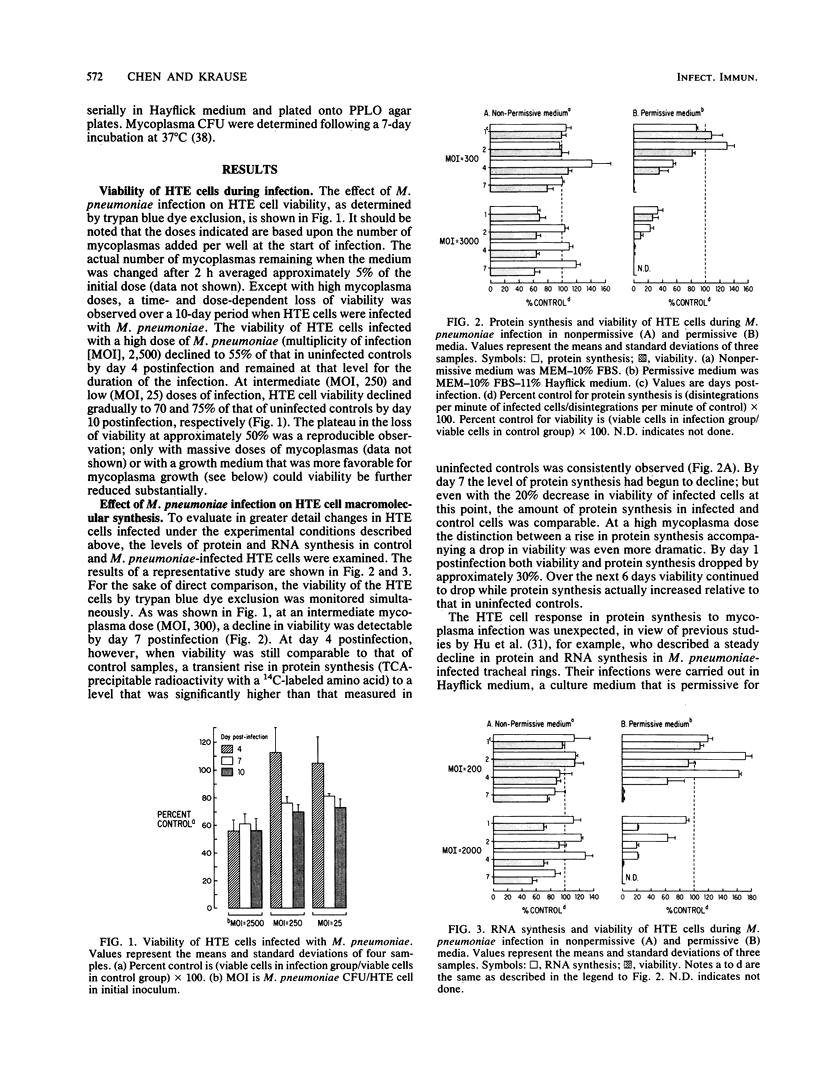



Selected References
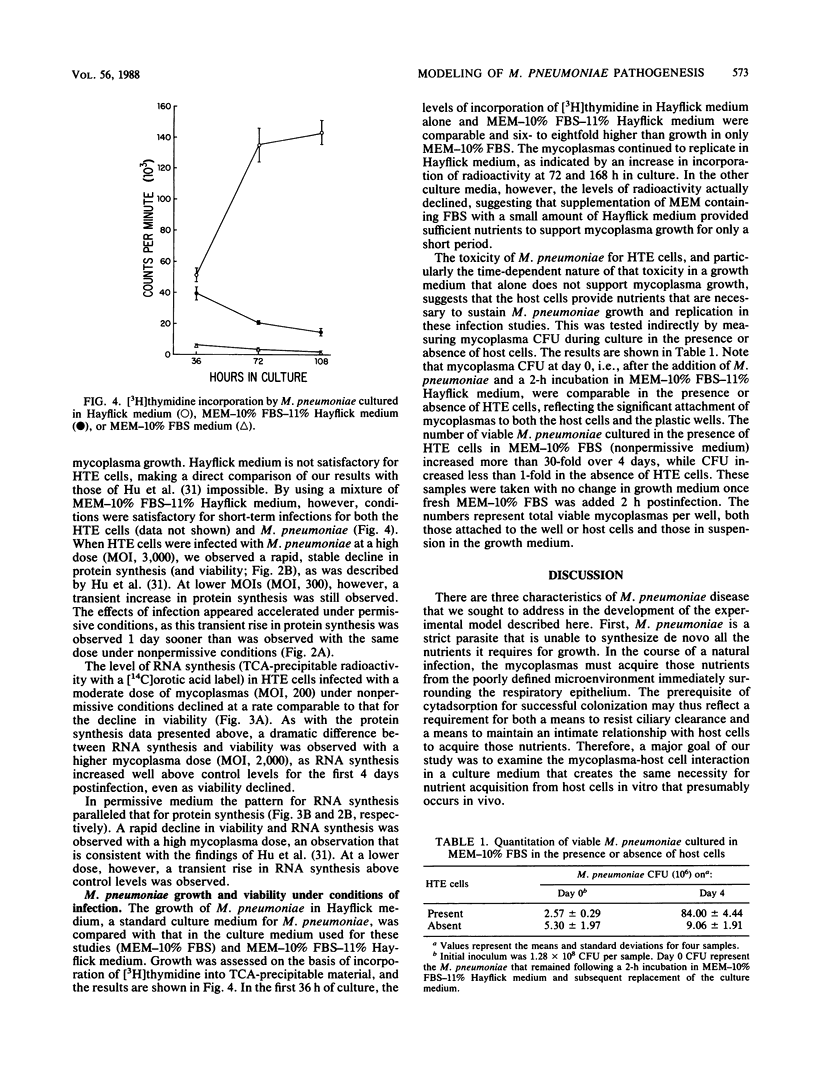
These references are in PubMed. This may not be the complete list of references from this article.
- Almagor M., Kahane I., Gilon C., Yatziv S. Protective effects of the glutathione redox cycle and vitamin E on cultured fibroblasts infected by Mycoplasma pneumoniae. Infect Immun. 1986 Apr;52(1):240–244. doi: 10.1128/iai.52.1.240-244.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almagor M., Kahane I., Yatziv S. Role of superoxide anion in host cell injury induced by mycoplasma pneumoniae infection. A study in normal and trisomy 21 cells. J Clin Invest. 1984 Mar;73(3):842–847. doi: 10.1172/JCI111279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almagor M., Yatziv S., Kahane I. Inhibition of host cell catalase by Mycoplasma pneumoniae: a possible mechanism for cell injury. Infect Immun. 1983 Jul;41(1):251–256. doi: 10.1128/iai.41.1.251-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camner P., Jarstrand C., Philipson K. Tracheobronchial clearance 5-15 months after infection with Mycoplasma pneumoniae. Scand J Infect Dis. 1978;10(1):33–35. doi: 10.3109/inf.1978.10.issue-1.07. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. C. Ultrastructural observations on cellular and subcellular aspects of experimental Mycoplasma pneumoniae disease. Infect Immun. 1980 Sep;29(3):1117–1124. doi: 10.1128/iai.29.3.1117-1124.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- DAJANI A. S., CLYDE W. A., Jr, DENNY F. W. EXPERIMENTAL INFECTION WITH MYCOPLASMA PNEUMONIAE (EATON'S AGENT). J Exp Med. 1965 Jun 1;121:1071–1086. doi: 10.1084/jem.121.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W., Clyde W. A., Jr, Glezen W. P. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J Infect Dis. 1971 Jan;123(1):74–92. doi: 10.1093/infdis/123.1.74. [DOI] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K., Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979 Jun;139(6):681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Dee Barden Stahl Y. Role of cell-associated pathogen metabolism in infection of tracheal explants by Mycoplasma pneumoniae. Med Microbiol Immunol. 1978 Oct 20;165(3):153–161. doi: 10.1007/BF02123172. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Johnson C. K., Cameron A. M. Cytotoxicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1974 Nov;10(5):1127–1134. doi: 10.1128/iai.10.5.1127-1134.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G. Oxygen consumption by trachea organ cultures infected with Mycoplasma pneumoniae. Infect Immun. 1975 Sep;12(3):544–549. doi: 10.1128/iai.12.3.544-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Intracellular levels of adenosine triphosphate in hamster trachea organ cultures exposed to Mycoplasma pneumoniae cells or membranes. In Vitro. 1977 Aug;13(8):510–516. doi: 10.1007/BF02615144. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Quantitative reduction of 2,3,4-triphenyl tetrazolium chloride by hamster trachea organ cultures: effects of Mycoplasma pneumoniae cells and membranes. Infect Immun. 1976 Jan;13(1):84–91. doi: 10.1128/iai.13.1.84-91.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G. Respiratory tract organ cultures to assay attachment and pathogenicity of mycoplasmas. Ann Microbiol (Paris) 1984 Jan-Feb;135A(1):33–38. doi: 10.1016/s0769-2609(84)80056-3. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Stahl Y. D. Role of adenine in pathogenesis of Mycoplasma pneumoniae infections of tracheal epithelium. Med Microbiol Immunol. 1978 May 26;165(1):43–55. doi: 10.1007/BF02121231. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Glycoprotein secretion by cultured hamster trachea epithelial cells: a model system for in vitro studies of mucus synthesis. In Vitro. 1980 Apr;16(4):320–329. doi: 10.1007/BF02618338. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980 Apr;16(4):313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. B., POTTER V. R. A survey of the metabolism of orotic acid in the rat. J Biol Chem. 1952 Mar;195(1):257–270. [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Isolation of mutants of Mycoplasma pneumoniae defective in hemadsorption. Infect Immun. 1979 Mar;23(3):903–906. doi: 10.1128/iai.23.3.903-906.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae. Infect Immun. 1979 May;24(2):468–475. doi: 10.1128/iai.24.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Clyde W. A., Jr, Baseman J. B. Characterization of hemadsorption-negative mutants of Mycoplasma pneumoniae. Infect Immun. 1981 Apr;32(1):127–136. doi: 10.1128/iai.32.1.127-136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Interaction of virulent Mycoplasma pneumoniae with hamster tracheal organ cultures. Infect Immun. 1976 Jul;14(1):217–224. doi: 10.1128/iai.14.1.217-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Mycoplasma pneumoniae proteins that selectively bind to host cells. Infect Immun. 1982 Jul;37(1):382–386. doi: 10.1128/iai.37.1.382-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr The interrelationship of virulence, cytadsorption, and peroxide formation in Mycoplasma pneumoniae. Proc Soc Exp Biol Med. 1969 Sep;131(4):1163–1167. doi: 10.3181/00379727-131-34061. [DOI] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J., Hayflick L., Perkins F. T. Modification of amino-acid concentrations induced by mycoplasmas in cell culture medium. Nat New Biol. 1971 Aug 25;232(34):242–244. doi: 10.1038/newbio232242a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. Mycoplasmas and cell cultures. Bacteriol Rev. 1971 Jun;35(2):206–227. doi: 10.1128/br.35.2.206-227.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch S., Gabridge M. G. Alterations in nucleotide content of human lung fibroblasts infected with Mycoplasma pneumoniae. Infect Immun. 1982 Nov;38(2):631–636. doi: 10.1128/iai.38.2.631-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch S., Gabridge M. G. Role of host cell metabolism in the pathogenesis of Mycoplasma pneumoniae infection. Infect Immun. 1981 Jan;31(1):174–181. doi: 10.1128/iai.31.1.174-181.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


