Abstract
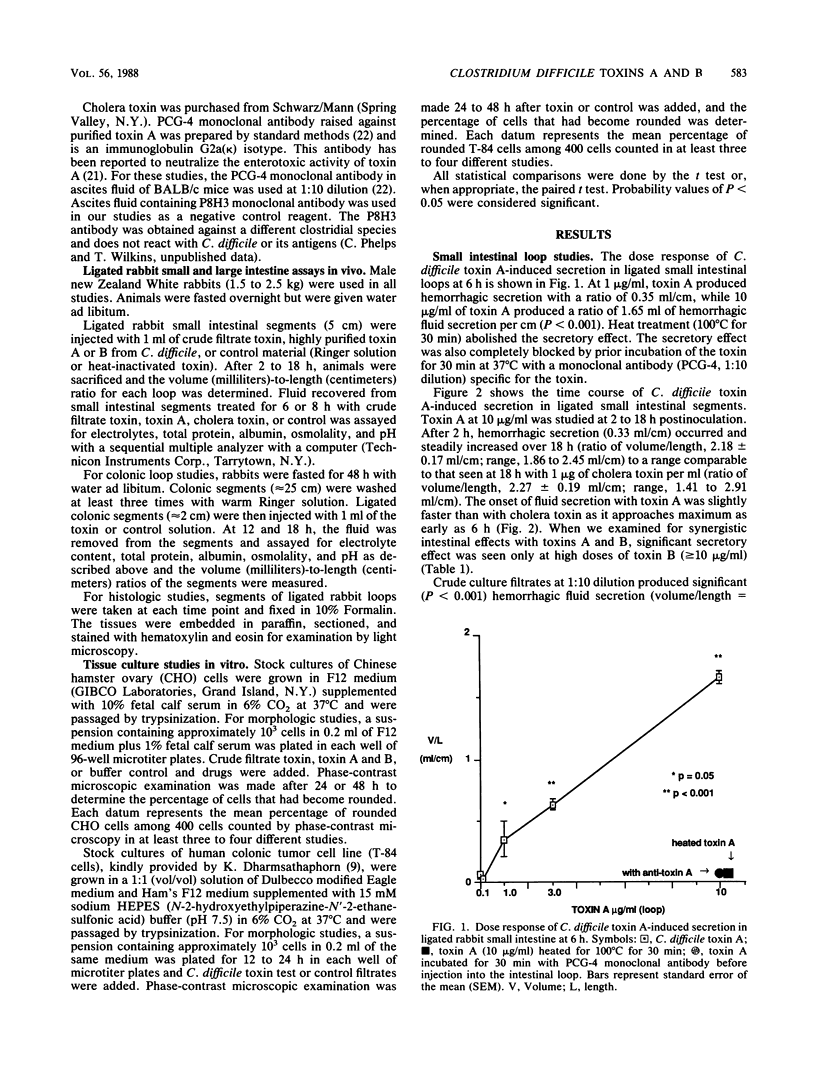
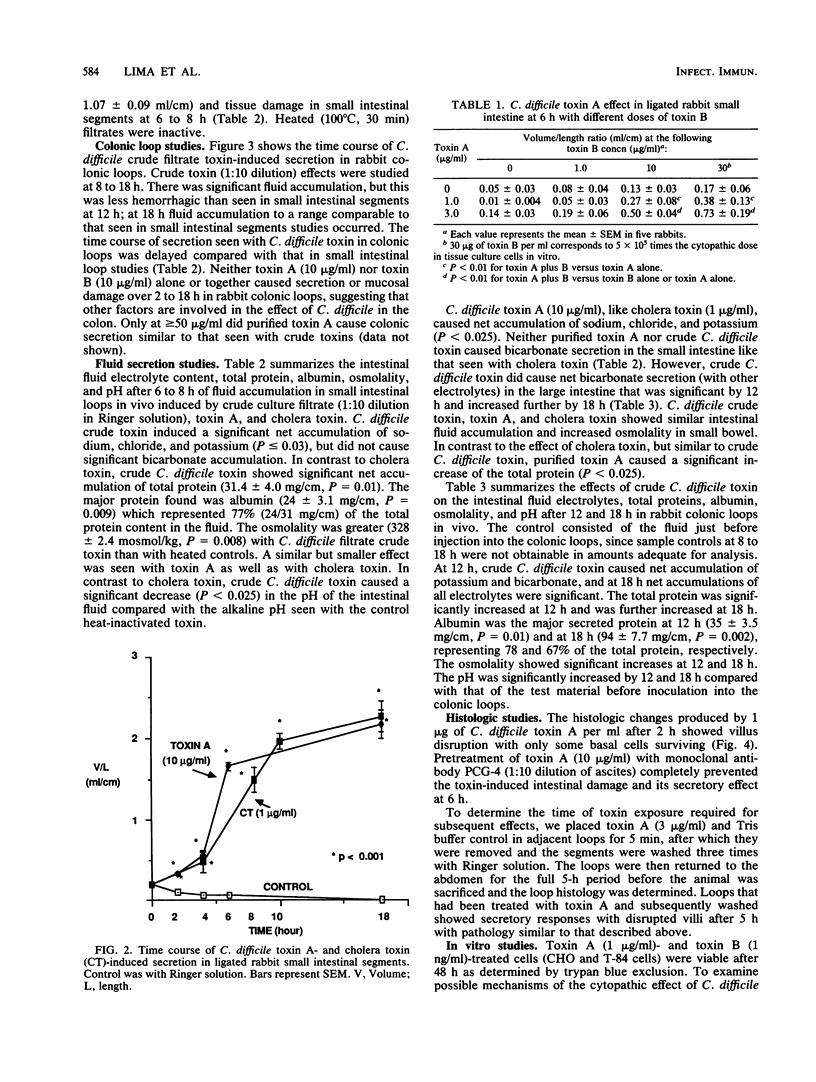
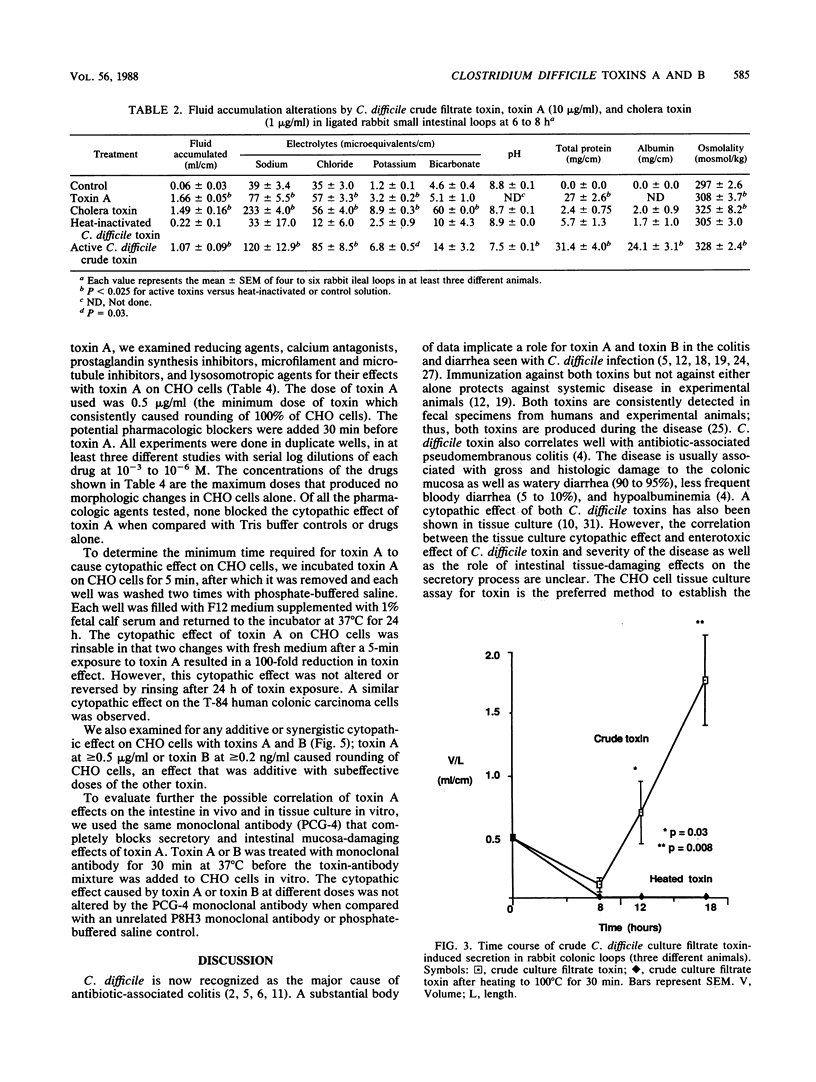
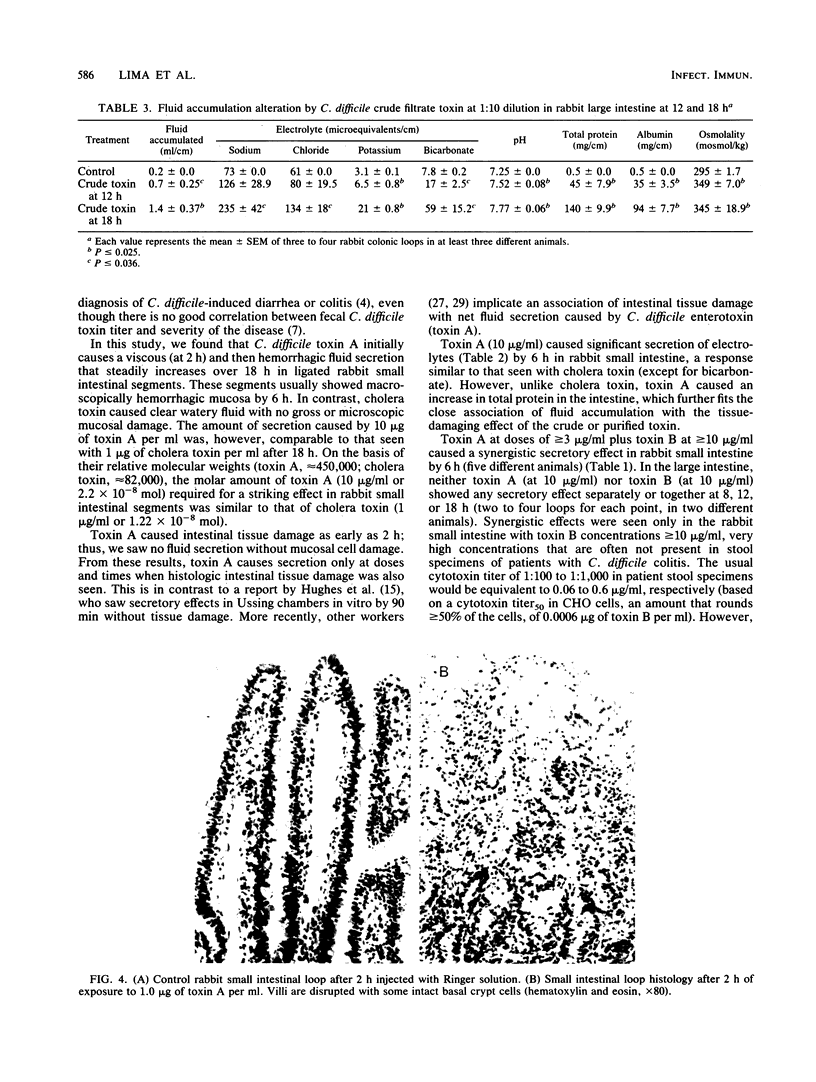
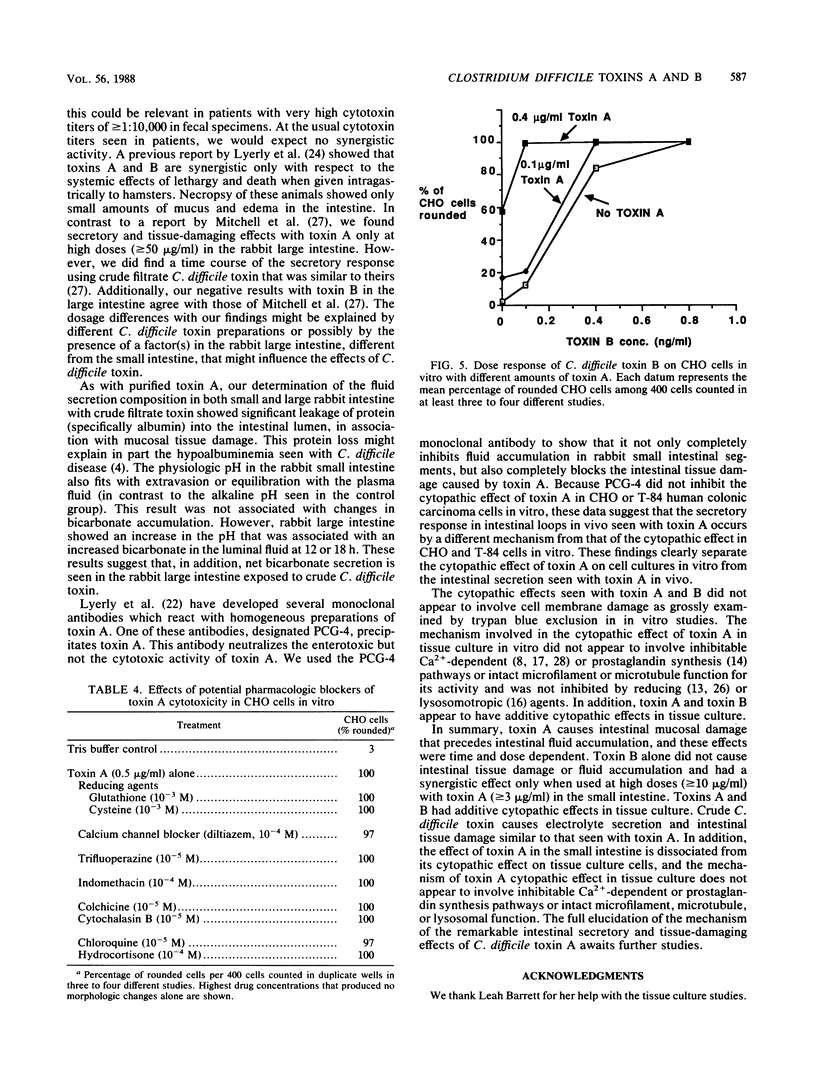
Clostridium difficile is recognized as the major cause of antibiotic-associated colitis. C. difficile produces two toxins, A (enterotoxin) and B (cytotoxin), that are implicated in the pathogenesis of the colitis. We examined the dose responses, time course, and synergism of these two toxins in ligated rabbit intestinal loops and in tissue culture. In rabbit small intestinal loops, toxin A caused histologically demonstrable intestinal tissue damage as early as 2 h. The secretory response greater than or equal to 8 h was similar to that of a cholera toxin control. The effect of toxin A on tissue damage or secretion was seen even if toxin was removed after 5 min. Purified toxin A caused significant net accumulation of sodium, chloride, potassium, and total protein and slightly increased osmolality of the fluid content at 6 h; these effects were similar to those caused by crude C. difficile culture filtrates containing toxins A and B. Crude C. difficile toxin caused fluid accumulation with a delayed time course in the rabbit large intestine, and in contrast to its effect in small intestine, crude toxin caused net accumulation of bicarbonate and increased pH. In tissue culture, toxin A caused a rounding up of CHO and T-84 colonic carcinoma cells. A monoclonal antibody (PCG-4) that has no effect on tissue culture cytotoxicity with toxins A and B completely inhibited the secretory and tissue-damaging effects in the intestine. Toxins A and B were synergistic in the gut only at high doses of toxin B (greater than or equal to 10 micrograms/ml), and they were additive in tissue culture. The cytopathic effect in tissue culture was not consistently associated with trypan blue uptake. The cytopathic effect of toxin A in tissue culture did not appear to involve inhibitable Ca2+-dependent or prostaglandin synthesis pathways or intact microfilament or microtubule function for its activity and was not inhibited by reducing or lysosomotropic agents. Our results suggest that toxins A and B have independent and distinct effects in vivo and in vitro.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Kobayashi T., Kono H., Watanabe K., Ueno K., Nozawa Y. Biochemical characterization and biologic actions of two toxins (D-1 and D-2) from Clostridium difficile. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S11–S20. doi: 10.1093/clinids/6.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G. Antimicrobial agents implicated in Clostridium difficile toxin-associated diarrhea of colitis. Johns Hopkins Med J. 1981 Jul;149(1):6–9. [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Taylor N. S., Chang T., Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2521–2526. doi: 10.1093/ajcn/33.11.2521. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G. Treatment of antibiotic-associated pseudomembranous colitis. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S235–S241. doi: 10.1093/clinids/6.supplement_1.s235. [DOI] [PubMed] [Google Scholar]
- Burdon D. W., George R. H., Mogg G. A., Arabi Y., Thompson H., Johnson M., Alexander-Williams J., Keighley M. R. Faecal toxin and severity of antibiotic-associated pseudomembranous colitis. J Clin Pathol. 1981 May;34(5):548–551. doi: 10.1136/jcp.34.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Shaffer S. J. Effects of Clostridium difficile toxin on tissue-cultured cells. J Infect Dis. 1980 Feb;141(2):218–222. doi: 10.1093/infdis/141.2.218. [DOI] [PubMed] [Google Scholar]
- Fekety R., Kim K. H., Brown D., Batts D. H., Cudmore M., Silva J., Jr Epidemiology of antibiotic-associated colitis; isolation of Clostridium difficile from the hospital environment. Am J Med. 1981 Apr;70(4):906–908. doi: 10.1016/0002-9343(81)90553-2. [DOI] [PubMed] [Google Scholar]
- Fernie D. S., Thomson R. O., Batty I., Walker P. D. Active and passive immunization to protect against antibiotic associated caecitis in hamsters. Dev Biol Stand. 1983;53:325–332. [PubMed] [Google Scholar]
- Greenberg R. N., Dunn J. A., Guerrant R. L. Reduction of the secretory response to Escherichia coli heat-stable enterotoxin by thiol and disulfide compounds. Infect Immun. 1983 Jul;41(1):174–180. doi: 10.1128/iai.41.1.174-180.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Murad F., Chang B., Robertson D. C., Guerrant R. L. Inhibition of Escherichia coli heat-stable enterotoxin by indomethacin and chlorpromazine. Infect Immun. 1980 Sep;29(3):908–913. doi: 10.1128/iai.29.3.908-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Warhurst G., Turnberg L. A., Higgs N. B., Giugliano L. G., Drasar B. S. Clostridium difficile toxin-induced intestinal secretion in rabbit ileum in vitro. Gut. 1983 Feb;24(2):94–98. doi: 10.1136/gut.24.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M. Shigella toxin(s): description and role in diarrhea and dysentery. Pharmacol Ther. 1981;15(3):403–438. doi: 10.1016/0163-7258(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Jortner B. S., Wilkins T. D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982 May;36(2):822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby J. M., Wilkins T. D. Production of antitoxins to two toxins of Clostridium difficile and immunological comparison of the toxins by cross-neutralization studies. Infect Immun. 1982 Jan;35(1):374–376. doi: 10.1128/iai.35.1.374-376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Toth J., Wilkins T. D. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun. 1986 Oct;54(1):70–76. doi: 10.1128/iai.54.1.70-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Ketley J. M., Haslam S. C., Stephen J., Burdon D. W., Candy D. C., Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986 Jan;27(1):78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen J., Redmond S. C., Mitchell T. J., Ketley J., Candy D. C., Burdon D. W., Daniel R. Clostridium difficile enterotoxin (toxin A): new results. Biochem Soc Trans. 1984 Apr;12(2):194–195. doi: 10.1042/bst0120194. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



