Abstract
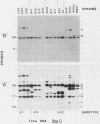
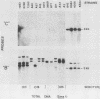
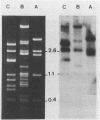
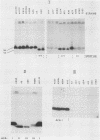
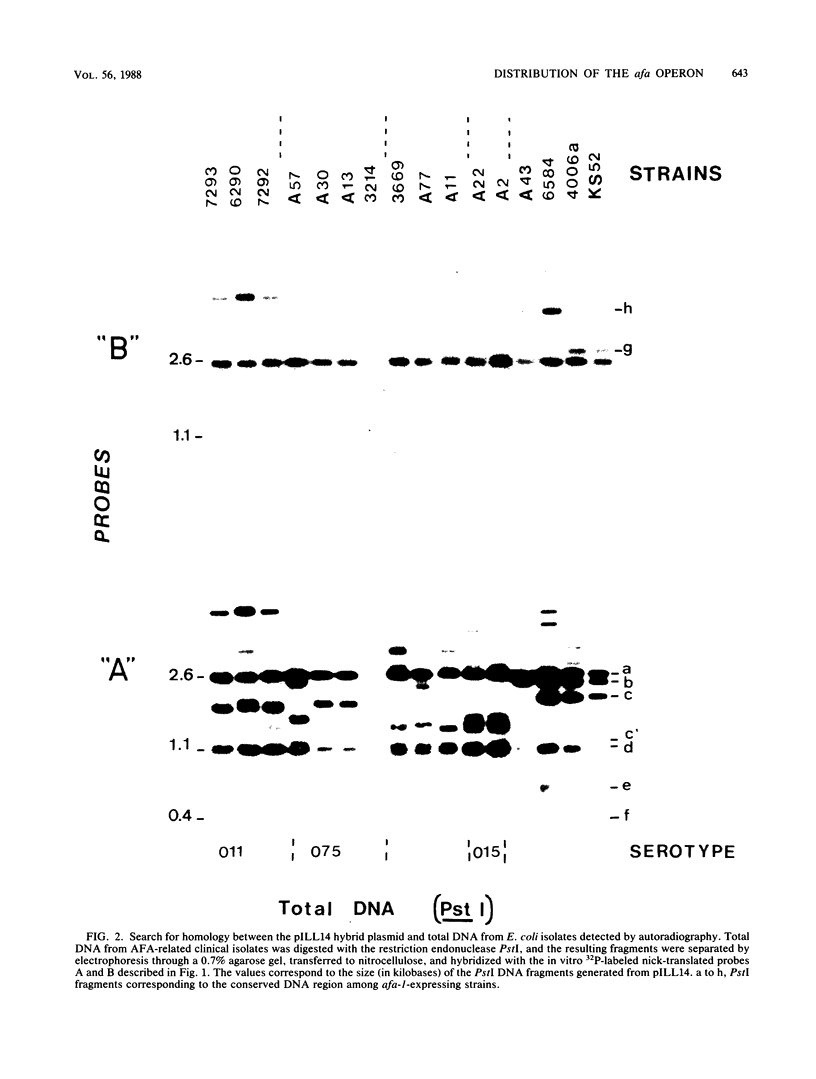
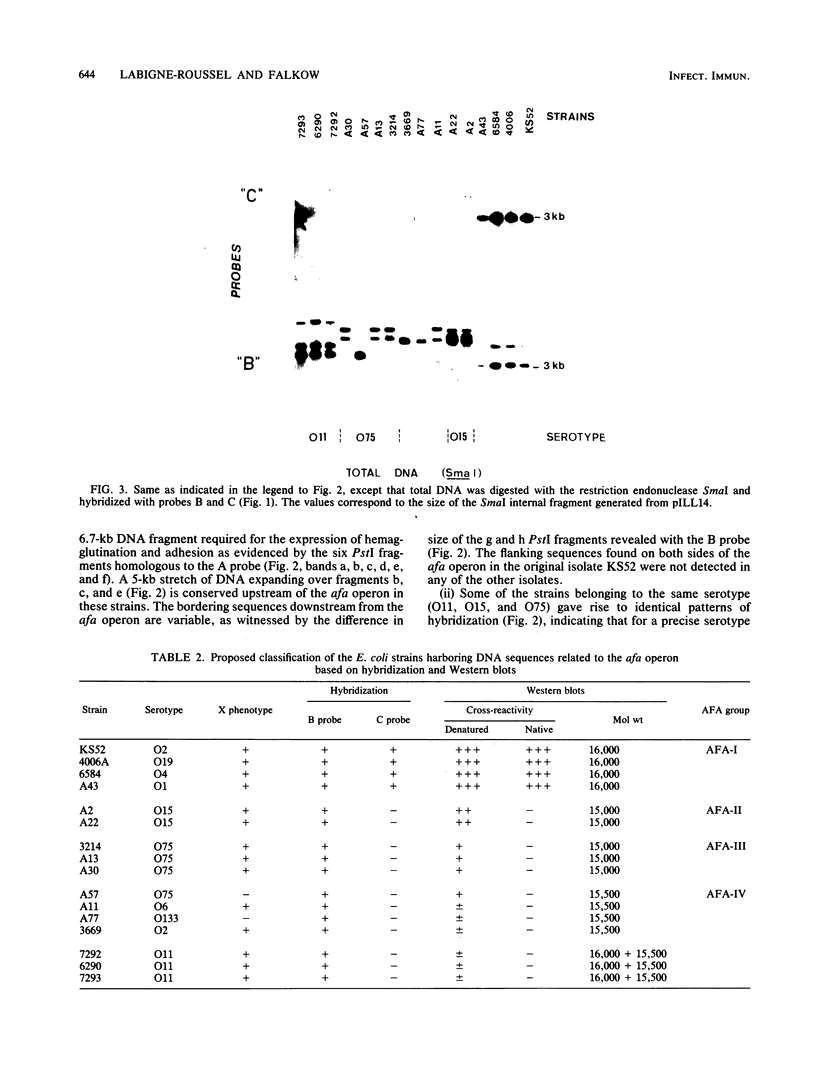
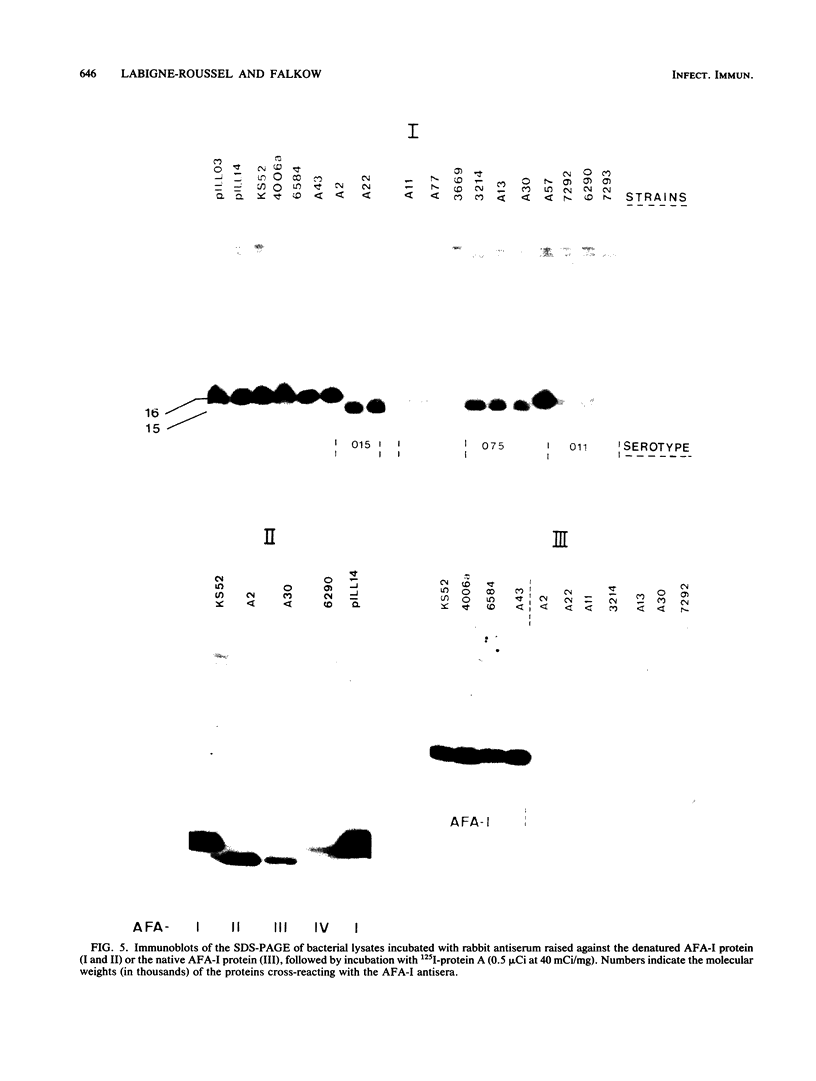
The afimbrial adhesin (AFA-I) from a pyelonephritic Escherichia coli isolate (KS52) is a mannose-resistant, P-independent, X-binding adhesin, expressed by the afa-1 operon. It is distinct from the E. coli X-binding adhesins with M and S specificity. A total of 138 E. coli isolates belonging to various serotypes, mostly from urinary tract infections, were screened for the presence of DNA sequences related to the afa operon and for the expression of an X-adhesin able to mediate mannose-resistant hemagglutination (MRHA) and adhesion to uroepithelial cells. Fifteen strains were shown to harbor DNA sequences related to the AFA-I-encoding operon, and 13 of them expressed an X-adhesin. Using as probes different DNA segments of the AFA-I-encoding operon in Southern experiments, we demonstrated that only three of these clinical isolates contained genetic determinants closely related to those identified in the original afa prototype strain (KS52): presence of the afaA, afaB, afaC, afaD, and afaE genes associated with the expression of a 16,000-dalton hemagglutinin-adhesin which strongly cross-reacted with AFA-I-specific antibodies. The other E. coli isolates harbored DNA sequences homologous to the afaA, afaB, afaC, and afaD genes, but lacked the sequence corresponding to the adhesin-producing gene afaE; Western blots allowed the detection of polypeptides (15,000, 15,500, or 16,000 daltons) in these strains which cross-reacted with variable intensity with antibodies raised against the denatured AFA-I protein, but did not cross-react with native AFA-I-specific antibodies. Following DNA cloning experiments from chromosomal DNA of two of those strains (A22 and A30), we demonstrated that although the AFA-related operon in A22 and A30 strains lacked the AFA-I adhesin-encoding gene, they synthesized a functional X-adhesin. Thus, strains A22 and A30 encode adhesins designated AFA-II and AFA-III, which were cloned on recombinant plasmids pILL72 and pILL61, respectively. Southern hybridization experiments and Western blot analyses of the 15 AFA-related strains demonstrate the heterogeneity of the genetic sequences encoding the structural adhesin and suggest the bases for the serological diversity of the AFA adhesins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buchanan K., Falkow S., Hull R. A., Hull S. I. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol. 1985 May;162(2):799–803. doi: 10.1128/jb.162.2.799-803.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Falkow S. Protein antigens from Staphylococcus aureus strains associated with toxic-shock syndrome. Science. 1981 Feb 20;211(4484):842–844. doi: 10.1126/science.7466361. [DOI] [PubMed] [Google Scholar]
- Collins J. Escherichia coli plasmids packageable in vitro in lambda bacteriophage particles. Methods Enzymol. 1979;68:309–326. doi: 10.1016/0076-6879(79)68022-9. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson G., Knutton S., Lam-Po-Tang M. K., McNeish A. S., Williams P. H. Adherence to human colonocytes of an Escherichia coli strain isolated from severe infantile enteritis: molecular and ultrastructural studies of a fibrillar adhesin. Infect Immun. 1987 Feb;55(2):393–402. doi: 10.1128/iai.55.2.393-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Then I., Müller D., Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Möllby R., Korhonen T., Winberg J., Cedergren B., Helin I., Hultberg H. Carbohydrate receptor structures recognized by uropathogenic E. coli. Scand J Infect Dis Suppl. 1982;33:52–60. [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D., David V., Lark D., Schoolnik G., Falkow S. Gene clusters governing the production of hemolysin and mannose-resistant hemagglutination are closely linked in Escherichia coli serotype O4 and O6 isolates from urinary tract infections. Infect Immun. 1984 Jan;43(1):353–358. doi: 10.1128/iai.43.1.353-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer L., Orndorff P. E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987 Feb;169(2):640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Serology of Escherichia coli fimbriae. Prog Allergy. 1983;33:80–105. [PubMed] [Google Scholar]
- Ott M., Hacker J., Schmoll T., Jarchau T., Korhonen T. K., Goebel W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986 Dec;54(3):646–653. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Rhen M., Klemm P., Korhonen T. K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-D-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984 Nov;46(2):401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]
- Walz W., Schmidt M. A., Labigne-Roussel A. F., Falkow S., Schoolnik G. AFA-I, a cloned afimbrial X-type adhesin from a human pyelonephritic Escherichia coli strain. Purification and chemical, functional and serologic characterization. Eur J Biochem. 1985 Oct 15;152(2):315–321. doi: 10.1111/j.1432-1033.1985.tb09200.x. [DOI] [PubMed] [Google Scholar]
- Williams P. H., Knutton S., Brown M. G., Candy D. C., McNeish A. S. Characterization of nonfimbrial mannose-resistant protein hemagglutinins of two Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):592–598. doi: 10.1128/iai.44.3.592-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]