Abstract
Uterine cancer is a common cause for cancer death in women and there is no effective therapy for metastatic disease. Thus, research is urgently needed to identify new therapeutic agents. We previously showed that all female HMGA1a transgenic mice develop malignant uterine tumors, indicating that HMGA1a causes uterine cancer in vivo. We also demonstrated that HMGA1a up-regulates cyclooxygenase-2 (COX-2) during tumorigenesis in this model. Similarly, we found that HMGA1a and COX-2 are overexpressed in human leiomyosarcomas, a highly malignant uterine cancer. While epidemiologic studies indicate that individuals who take cyclooxygenase inhibitors have a lower incidence of some tumors, these inhibitors have not been evaluated in uterine cancer. Here, we show that HMGA1a mice on sulindac (a COX-1/COX-2 inhibitor) have significantly smaller uterine tumors than controls. To determine if cyclooxygenase inhibitors are active in human uterine cancers that overexpress HMGA1a, we treated cultured cells with sulindac sulfide or celecoxib (a specific COX-2 inhibitor). Both drugs block anchorage-independent growth in high-grade human uterine cancer cells that overexpress HMGA1a (MES-SA cells). In contrast, neither inhibitor blocked transformation in cells that do not overexpress HMGA1a. Moreover, xenograft tumors from MES-SA cells were significantly inhibited in mice on sulindac. More strikingly, no tumors formed in mice on celecoxib. These preclinical studies suggest that cyclooxygenase inhibitors could play a role in preventing tumor onset or progression in uterine cancers with dysregulation of the HMGA1a-COX-2 pathway. Importantly, these drugs have lower toxicity than chemotherapeutic agents used to treat advanced stage uterine cancers.
Keywords: HMGA1, cyclooxygenase, uterine cancer
Introduction
Despite recent advances in our understanding of the molecular abnormalities associated with uterine cancer, these discoveries have not been translated into better therapy for patients (1-3). Uterine cancer is the most common cancer of the female genital tract and the fourth most frequent cause for cancer death in women in the United States (1-2). These cancers include carcinomas, sarcomas, and mixed epithelialmesenchymal tumors. For all subtypes, the only available treatment is hysterectomy. There is no effective therapy once these tumors have spread outside the uterus; adjuvant therapy for metastatic disease does not enhance survival. Thus, further study is needed, not only to identify chemotherapeutic agents to treat uterine cancer after it has developed, but also to discover new strategies to prevent or delay disease progression. Preclinical studies of therapeutic interventions for uterine cancer recently became possible with the generation of HMGA1a transgenic mice, which develop malignant uterine tumors with complete penetrance (4). These tumors recapitulate salient histologic and molecular features found in human uterine sarcomas (4). At the molecular level, we showed that HMGA1a up-regulates cyclooxygenase-2 (COX-2) during uterine tumorigenesis in this model (4). We also found that high-grade human leiomyosarcomas overexpress both HMGA1a and COX-2, similar to our transgenic mice (4). COX-2 is a key enzyme involved in prostaglandin formation, which leads to signal transduction, inflammation, and mitogenesis, depending upon the cellular context (5). While COX-2 has been well studied in human cancers of epithelial origin, such as gastrointestinal, breast, and prostate (5), there are no studies evaluating its functional role in uterine cancer. We therefore sought to determine if cyclooxygenase inhibitors could block uterine tumorigenesis using both our transgenic mice and human uterine cancer xenografts as model systems. Although additional studies are needed, our findings indicate that cyclooxygenase inhibitors could play a role in preventing the onset or progression of selected uterine cancers in humans.
Materials and Methods
Drugs
Celecoxib (800 ppm, LKT Laboratories, Inc.) or sulindac (320 ppm, Sigma-Aldrich) were mixed with pelleted, purified rodent diet AIN-76A (Dyets, Inc.). For in vitro studies, sulindac sulfide, sulindac sulfone (Sigma-Aldrich) and celecoxib were prepared in DMSO and added to media. DMSO alone was used for controls.
Cell lines and in vitro studies
Human uterine cancer cell lines MES-SA and SK-UT-1 obtained from ATCC were grown as recommended. MES-SA cells (6) are derived from a poorly differentiated uterine cancer that arose as a recurrent tumor five months after hysterectomy for a malignant mixed mullerian tumor (carcinosarcoma). SK-UT-1 cells are derived from the sarcomatous region of a grade III malignant mixed mullerian tumor. Both cells lines are designated as sarcoma cells (ATCC). Cell proliferation was assessed using the CellTiter Cell Proliferation Assay (Promega). Anchorage-independent growth was assessed as previously described (4).
Mice
The construction of the transgenic mice was previously described (4, 7). Briefly, the cDNA encoding murine HMGA1a (previously HMG-I; ref. 8) was cloned into the vector pHSE3' (9). This construct was cleaved with XhoI to release a DNA fragment containing the HMGA1a coding sequence flanked by the H-2K promoter and immunoglobulin μ intronic enhancer (7). The transgene was injected into fertilized eggs from B6C57/SJL females and maintained by mating hemizygous mice with wild-type B6C57 mice (7). This construct was previously shown to drive transgene expression in T and B lymphoid cells (7, 9). In addition, we showed that the transgene is also overexpressed in the uteri of all female mice (4). As previously reported, all mice develop lymphoid malignancies (7) and all females develop uterine tumors by 8-10 months (4). The females exhibit mild uterine enlargement and abnormal histopathologic findings at necropsy as early as 8-10 weeks with large sarcomas by 8-10 months (4). Mouse experiments were approved by the Animal Care and Use Committee according to NIH guidelines.
Chemoprevention study
At 8 weeks of age, HMGA1a transgenic mice (10 in each arm) were randomly assigned to one of three groups: control, sulindac, or celecoxib. Body weights were monitored weekly. Mice were sacrificed at either 29 or 36 weeks of age (5 in each group). Data were analyzed by Student's t test; p < 0.05 was considered significant.
Uterine xenograft studies
Uterine cancer cells (5.0 × 106) were injected subcutaneously into nude mice as described (6, 8). Mice were fed celecoxib, sulindac or control food starting 3 days before injections. Mice were sacrificed when tumors reached a maximum diameter of 1.5 cm (after 11-14 days) in the control group. Tumor volumes were estimated as length × width2/2 (6). The proportion of mice with tumors in each group was compared by the exact test of the null hypothesis of equal proportions between two groups.
Histology
Gene expression Analysis
RNA extraction, reverse transcription and quantitative Real Time PCR (QRT-PCR) were performed as previously described (4).
Results
Sulindac inhibits uterine tumorigenesis in theHMGA1amice
We used our HMGA1a mice for the initial preclinical studies because they develop uterine cancer that recapitulates histologic and molecular features characteristic of human uterine sarcomas (4). To determine if cyclooxygenase inhibitors could prevent the development or progression of uterine cancer, the mice were fed a diet supplemented with either sulindac, a COX-1/COX-2 inhibitor (320 ppm, n = 10), celecoxib, a specific COX-2 inhibitor (800 ppm; n = 10), or control (n = 10), starting at 8 weeks of age. The doses were based on published reports that showed antitumor efficacy in mice (10-12). There was no significant difference in food intake or weight gain in each treatment arm over the study period (Supplementary Fig. S1).
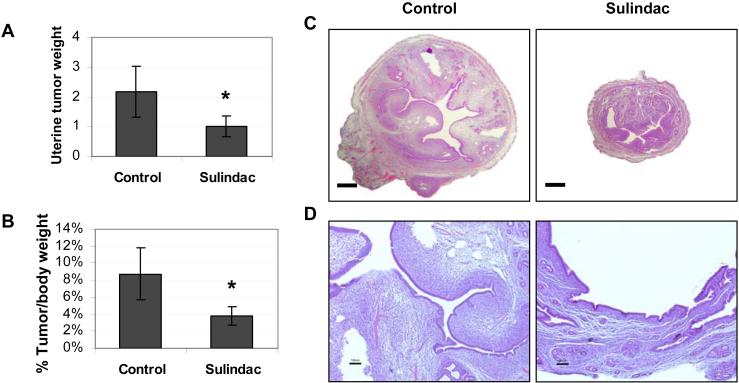
At 29 weeks of age (Fig 1A), there were significantly smaller uterine tumors in the sulindac arm (1.01 ± 0.34 grams vs. 2.17 ± 0.84 grams; p = 0.024, n = 5). To control for differences in body weight, the tumor size was expressed as percentage of body weight (Fig. 1B), and the difference was even more significant (3.85 ± 1.09 % vs. 8.75 ± 3.05 %; p = 0.014, n = 5). Histopathologic examination of the uterine tumors on sulindac showed a decrease in volume, edema, and hypercellularity of the endometrial stroma. The uterus in the treated mouse also lacks the polypoid proliferation that protrudes into the uterine cavity in the untreated mouse (Fig. 1C-D). The celecoxib arm did not show statistically significant differences compared to controls at 29 weeks of age (Supplementary Fig. S2).
Figure 1. Sulindac inhibits uterine tumorigenesis in the HMGA1a mice.
A, At 29 weeks the uterine tumors from the transgenic mice treated with sulindac were significantly smaller that those of control mice. The bars show the mean uterine weight (grams) ± standard deviation (SD) from 5 mice in each group (*, p = 0.024).
B, In this panel, the uterine tumor weight was divided by the total body weight. Again, tumors in mice treated with sulindac were significantly smaller (*, p = 0.014).
C, Transverse section through the uterine horn from a representative HMGA1a transgenic mouse treated with sulindac (left panels) compared to an untreated mouse (right panels; bars, 500 μm). The uterine horn of the untreated mouse is expanded and filled by an intracavitary stromal proliferation. In contrast, the horn and intracavitary lesion are markedly reduced in size.
D, Higher magnification of the uterine tumors from an HMGA1a mouse treated with sulindac compared to control (bars, 100 μm) demonstrates a polypoid herpercellular and edematous stromal proliferation with intimately associated glandular epithelium in the untreated mouse. In the treated mouse, the lesion is reduced to an attenuated endometrium lacking the polypoid proliferation.
At 36 weeks, tumors were slightly smaller in both the sulindac arm (2.50 grams ± 1.96 grams or 9.05% ± 6.53%; n = 5) and celecoxib arm (2.47 grams ± 1.82 grams or 8.63% ± 6.13%; n = 5) compared to controls (3.42 grams ± 2.24 grams or 12.65% ± 8.54%), although the differences were not statistically significant (Supplementary Fig. S2). At this later time point (36 weeks), it is possible that the oncogenic influence from the potent HMGA1a transgene allows tumor cells to escape the inhibitory effects of the drugs. Of note, the inhibitors had no effect on the lymphoid malignancies at the time points studied. Examination of tumor burden by splenic weights, histopathologic examination, and fluorescence activated cell sorting showed no difference in the leukemic tumor burdens (data not shown).
To determine if sulindac has a direct effect on HMGA1a expression levels in the tumors, we assessed HMGA1a mRNA levels in the uterine tumor tissue at necropsy in the mice in the sulindac or control arms. By QRT-PCR, we found no difference in the levels of HMGA1a mRNA in all groups tested at 29 weeks or 36 weeks, indicating that sulindac did not directly influence the expression of the transgene (Supplementary Fig. S3).
Sulindac sulfide and celecoxib block anchorage-independent cell growth in human uterine cancer cellsin vitro
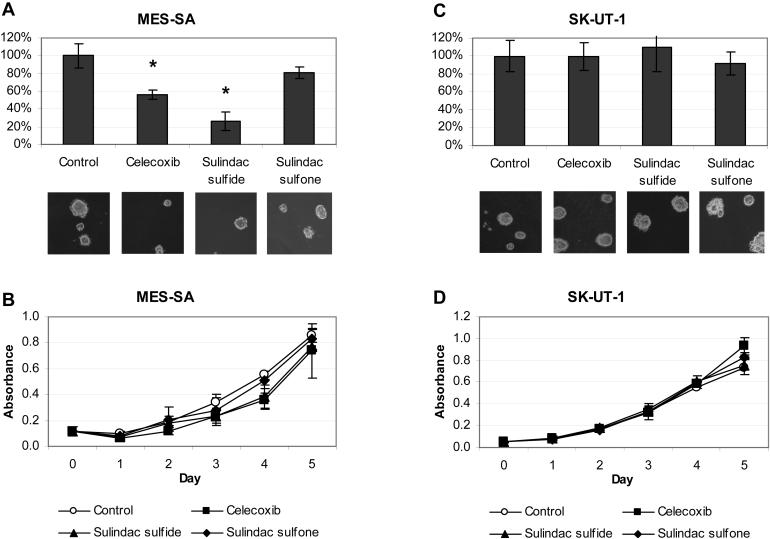
Because sulindac was effective in blocking tumor growth in our HMGA1a mouse model for uterine cancer, we next sought to determine if cyclooxygenase inhibitors interfere with transformation in vitro in high-grade human uterine cancer cells with high levels of HMGA1a expression. To this end, we assessed anchorage-independent cell growth in MES-SA cells, which have high levels of HMGA1a (4), and SK-UT-1 cells, which have low levels of HMGA1a (See Supplementary Fig. S4 for comparison of HMGA1a expression). Because sulindac is a pro-drug that is metabolized to its active forms (sulindac sulfide and sulindac sulfone) in vivo, we used the active metabolites for these studies. Sulindac sulfide is a non-steroidal anti-inflammatory compound with COX-1/COX-2 inhibitory effects; sulindac sulfone does not possess cyclooxygenase inhibitory activity (13). Drug concentrations that can be achieved in the plasma of patients on standard doses of sulindac or celecoxib were incubated with uterine cancer cells (14-16). Strikingly, we found that anchorage-independent cell growth was significantly inhibited by sulindac sulfide (50 μmol/L; p = 0.03) or celecoxib (10 μmol/L; p = 0.03) in the MES-SA cells (Fig. 2A). In contrast, sulindac sulfone (50 μmol/L) had no effect on foci formation (Fig. 2A). Moreover, cellular proliferation was not affected by any of these compounds (Fig 2B), indicating that the concentrations were not toxic, but rather, inhibit foci formation through a transformation-specific mechanism. In contrast to the MES-SA cells, the cyclooxygenase inhibitors had no effect on foci formation in the SK-UT-1 cells (Fig. 2C-D). Taken together these results indicate that the COX-2 inhibitors are effective in vitro in blocking anchorage-independent cell growth in high-grade uterine cancer cells that overexpress HMGA1a.
Figure 2. Sulindac sulfide or celecoxib block anchorage-independent cell growth in human uterine cancer cells overexpressing HMGA1a.
A, MES-SA uterine cancer cells form fewer foci on methylcellulose in the presence of celcoxib (10 μmol/L) and sulindac sulfide (50 μmol/L) compared to vehicle control.
B, Cellular proliferation of MES-SA cells is not affected by the sulindac metabolites or celecoxib.
C, In SK-UT-1 uterine cancer cells, foci formation is not affected by sulindac metabolites or celecoxib.
D, Cellular proliferation is also not affected by sulindac metabolites or celecoxib in the SK-UT-1 cells.
Graphs show the mean ± SD from at least two experiments performed in triplicate. *, p < 0.05.
Sulindac and celecoxib block tumorigenesis in human uterine cancer xenografts overexpressingHMGA1a
Next, we sought to determine if the cyclooxygenase inhibitors could block tumorigenesis in the human uterine cancer cells that express high levels of HMGA1ain vivo. To this end, we injected MES-SA human uterine cancer cells subcutaneously into nude mice fed control diet or diet supplemented with sulindac or celecoxib. Large subcutaneous tumors formed in all (6/6) nude mice on control food after only 14 days (Table 1, Fig 3A). As expected, the MES-SA xenograft tumors overexpressed HMGA1a (Fig 3B). Tumor volumes ranged from 62.5 to 2025 mm3 (mean volume: 561 ± 735 mm3). In contrast, sulindac significantly inhibited tumor formation (p = 0.02). Only one small tumor (100 mm3) formed in the 6 nude mice treated with sulindac. Moreover, mice treated with celecoxib developed no tumors (0/6; p < 0.0001). These findings demonstrate that sulindac, which inhibits both COX-1 and COX-2, or celecoxib, a specific COX-2 inhibitor, both block tumorigenesis in our high HMGA1a expressing MES-SA uterine xenograft model (Table 1, Fig. 3). Interestingly, neither inhibitor was effective on SK-UT-1 cells (Supplementary Table 1). HMGA1a expression in the SK-UT-1 xenografts was low (Fig. 3B), like we observed in the in vitro cell cultures (Supplementary Fig. S4). Taken together, these experiments indicate that sulindac and celecoxib are effective in blocking tumorigenesis in uterine xenografts from high-grade uterine cancer cells that overexpress HMGA1a.
Table 1.
Effect of sulindac or celecoxib on xenograft tumor formation in nude mice injected with MES-SA (high HMGA1a) human uterine cancer cells
| Tumor volume (mm3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell line | Treatment | Tumor # | 1 | 2 | 3 | 4 | 5 | 6 |
| MES-SA | ||||||||
| Control | 6/6 (100%) | 125 | 270 | 384 | 62.5 | 500 | 2025 | |
| Sulindac | 1/6 (17%)* | 100 | - | - | - | - | - | |
| Celecoxib | 0/6 (0%)** | - | - | - | - | - | - | |
p = 0.02
p < 0.0001
Figure 3. Sulindac and celecoxib block tumorigenesis in human uterine cancer xenografts overexpressing HMGA1a.
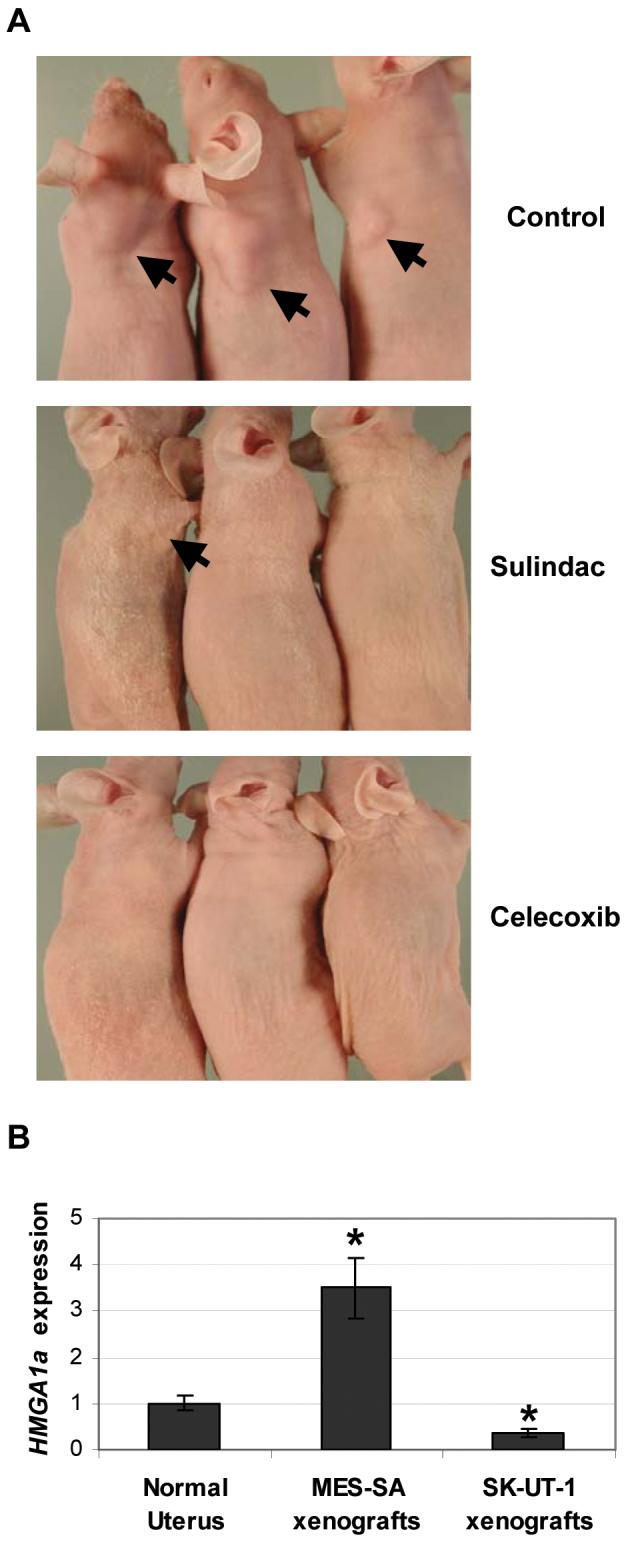
A, representative transgenic mice treated with control food or food supplemented with sulindac or celecoxib.
B, MES-SA xenografts overexpress HMGA1a mRNA compared to normal uterus or SK-UT-1 xenografts. The bars represent the mean ± SD from at least two experiments performed in triplicate. *, p < 0.05
Discussion
Because uterine cancer is common and often fatal, novel strategies to treat or prevent tumor progression in these malignancies will benefit women's health worldwide (1, 2). Although uterine sarcomas comprise only about 5% of all uterine cancers, they are more aggressive and have a worse prognosis than other uterine cancers (3). Leiomyosarcomas constitute about 30% of uterine sarcomas and have a 5 year survival of less than 20% for tumors that extend beyond the corpus uteri. Malignant mixed mullerian tumors (MMMTs or carcinosarcomas) represent another high-grade uterine cancer comprised of both malignant mesenchymal (sarcomatous) and epithelial (carcinomatous) components. MMMTs represent approximately 5% of all uterine cancers and have a 5 year survival of 40-50% for all stages (2, 3). The ability to perform preclinical trials relevant to these types of aggressive uterine cancer was recently advanced by the development of the HMGA1a mice, which develop malignant uterine tumors with complete penetrance by 8-10 months of age (4). These mice express HMGA1a in the uterus at levels 5 to 15-fold above those observed in normal uteri and up-regulate COX-2 during uterine tumorigenesis (4). Similarly, our previous study showed that human leiomyosarcomas overexpress HMGA1a by 5 to 20-fold above normal uterine tissue and COX-2 is also up-regulated in these tumors (4). Other mouse models for uterine sarcomas have relied on exposure to carcinogenic agents (17) or overexpression of a viral oncogene (18). Our model is unique because the initiating genetic lesion (overexpression of the HMGA1a oncogene) occurs in diverse, high-grade human uterine cancers (4). Using this model, we showed that sulindac blocks uterine tumor growth. Moreover, we demonstrated that both sulindac and celecoxib were effective in blocking transformation in human uterine cancer cells with high levels of HMGA1a, both in vitro and in vivo. Our results suggest that uterine cancers characterized by up-regulation of HMGA1a will respond to cyclooxygenase inhibitors.
Previous studies have shown that cyclooxygenase inhibitors have pleiotropic anti-tumor effects, although these investigations have focused primarily on tumors of epithelial origin. Most work in this area has been directed at gastrointestinal tumors with both in vitro and in vivo studies demonstrating anti-tumor efficacy with cyclooxygenase inhibitors (5, 12). More recently, epidemiologic studies have shown that individuals who take aspirin, which blocks both COX-1 and COX-2 function, have lower incidences of diverse tumors. Similarly, celecoxib has been shown to have anti-tumor effects in patients with lung and other cancers (5, 12). Enthusiasm for COX-2 inhibitors has been dampened, however, by the recent reports of cardiovascular toxicity in rare cases. Nonetheless, a recent clinical trial with lung cancer demonstrated benefit in selected patients on celecoxib and no cardiovascular toxicity (19). Thus, the current challenge is to identify selected patients who could benefit from COX-2 inhibitors and to develop next generation agents that lack toxicity. Because uterine cancers that extend beyond the uterus have a dismal prognosis, these patients could benefit from cyclooxygenase inhibitors. The mechanism of action of cyclooxygenase inhibitors is not completely understood, although studies indicate that several pathways may be affected, including angiogenesis, invasion, and inhibition of apoptosis (5). Our in vitro and in vivo studies showed that sulindac and celecoxib were efficacious in blocking transformation in uterine cancer cells that overexpress HMGA1a, suggesting that HMGA1a could serve as a marker to identify uterine cancers that may respond to cyclooxygenase inhibitors. The inhibition of anchorage-independent cell growth by celecoxib, sulindac sulfide, and not sulindac sulfone, is consistent with a COX-2-dependent mechanism of action in our in vitro studies.
In summary, we show that our HMGA1a mouse model is a useful tool for preclinical therapeutic studies. Furthermore, our studies suggest that COX-2 inhibitors could play a role in preventing tumor development or progression in women with high-grade uterine cancers that up-regulate HMGA1a. Importantly, COX-2 inhibitors have lower toxicity than the chemotherapeutic agents currently used to treat advanced stage uterine cancers. Moreover, these tumors are typically unresponsive to therapy. Thus, future studies are warranted to investigate the role of cyclooxygenase inhibitors in uterine cancers.
Supplementary Material
Acknowledgements
We thank Ms. Shamayra Smail for help with the RNA extractions. We would also like to acknowledge the numerous authors of papers we could not reference secondary to space limitations.
Grant Support: NIH R21SG108797, the Leukemia & Lymphoma Scholar Award 1694-06, the American Cancer Society, and Alex Lemonade Stand Foundation Award (L. M. S. Resar), Flight Attendant Medical Research Institute (F. Di Cello), Gynecologic Cancer Foundation (J. Hillion)
Abbreviations
- HMGA1a
high mobility group A1a
- QRT-PCR
quantitative real time polymerase chain reaction
- COX-2
cyclooxygenase-2
References
- 1.Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–8. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Ronnett BM, Zaino RJ, Ellenson LH, Kurman RJ. Endometrial carcinoma. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. 5th ed. Springer-Verlag; New York: 2002. pp. 501–59. [Google Scholar]
- 3.Pautier P, Genestie C, Rey A, et al. Analysis of clinicopathologic prognostic factors for 157 uterine sarcomas and evaluation of a grading score validated for soft tissue sarcoma. Cancer. 2000;88:1425–31. [PubMed] [Google Scholar]
- 4.Tesfaye A, Di Cello F, Hillion J, et al. The high-mobility group A1 gene up-regulates cyclooxygenase 2 expression in uterine tumorigenesis. Cancer Res. 2007;67:3998–04. doi: 10.1158/0008-5472.CAN-05-1684. [DOI] [PubMed] [Google Scholar]
- 5.Meric JB, Rottey S, Olaussen K, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Harker WG, MacKintosh FR, Sikic BI. Development and characterization of a human sarcoma cell line, MES-SA, sensitive to multiple drugs. Cancer Res. 1983;43:4943–50. [PubMed] [Google Scholar]
- 7.Xu Y, Sumter TF, Bhattacharya R, et al. The HMG-I oncogene causes highly penetrant, aggressive lymphoid malignancy in transgenic mice and is overexpressed in human leukemia. Cancer Res. 2004;64:3371–5. doi: 10.1158/0008-5472.CAN-04-0044. [DOI] [PubMed] [Google Scholar]
- 8.Wood LJ, Maher JF, Bunton TE, Resar LM. The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–61. [PubMed] [Google Scholar]
- 9.Malek S, Dordai DL, Reim J, Dintziz H, Desiderio S. Malignant transformation of early lymphoid progenitors in mice expressing an activated Blk tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7351–6. doi: 10.1073/pnas.95.13.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004;10:7727–37. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- 11.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–70. [PubMed] [Google Scholar]
- 12.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 13.Piazza GA, Alberts DS, Hixson LJ, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–15. [PubMed] [Google Scholar]
- 14.Dvory-Sobol H, Kazanov D, Liberman E, et al. MF tricyclic and sulindac retard tumor formation in an animal model. Int J Cancer. 2006;118:11–6. doi: 10.1002/ijc.21218. [DOI] [PubMed] [Google Scholar]
- 15.Stempak D, Gammon J, Halton J, Champagne M, Koren G, Baruchel S. Modulation of celecoxib pharmacokinetics by food in pediatric patients. Clin Pharmacol Ther. 2005;77:226–8. doi: 10.1016/j.clpt.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Ravis WR, Diskin CJ, Campagna KD, Clark CR, McMillian CL. Pharmacokinetics and dialyzability of sulindac and metabolites in patients with end-stage renal failure. J Clin Pharmacol. 1993;33:527–34. doi: 10.1002/j.1552-4604.1993.tb04699.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitsumori K, Onodera H, Shimo T, et al. Rapid induction of uterine tumors with p53 point mutations in heterozygous p53-deficient CBA mice given a single intraperitoneal administration of N-ethyl-N-nitrosourea. Carcinogenesis. 2000;21:1039–42. doi: 10.1093/carcin/21.5.1039. [DOI] [PubMed] [Google Scholar]
- 18.Politi K, Szabolcs M, Fisher P, Kljuic A, Ludwig T, Efstratiadis A. A mouse model of uterine leiomyosarcoma. Am J Pathol. 2004;164:325–36. doi: 10.1016/S0002-9440(10)63122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csiki I, Morrow JD, Sandler A, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: A phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–40. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.