Abstract
Returning to normal pH after acidosis, similar to reperfusion after ischemia, is prone to arrhythmias. The type and mechanisms of these arrhythmias have never been explored and were the aim of the present work. Langendorff-perfused rat/mice hearts and rat-isolated myocytes were subjected to respiratory acidosis and then returned to normal pH. Monophasic action potentials and left ventricular developed pressure were recorded. The removal of acidosis provoked ectopic beats that were blunted by 1 μM of the CaMKII inhibitor KN-93, 1 μM thapsigargin, to inhibit sarcoplasmic reticulum (SR) Ca2+ uptake, and 30 nM ryanodine or 45 μM dantrolene, to inhibit SR Ca2+ release and were not observed in a transgenic mouse model with inhibition of CaMKII targeted to the SR. Acidosis increased the phosphorylation of Thr17 site of phospholamban (PT-PLN) and SR Ca2+ load. Both effects were precluded by KN-93. The return to normal pH was associated with an increase in SR Ca2+ leak, when compared with that of control or with acidosis at the same SR Ca2+ content. Ca2+ leak occurred without changes in the phosphorylation of ryanodine receptors type 2 (RyR2) and was blunted by KN-93. Experiments in planar lipid bilayers confirmed the reversible inhibitory effect of acidosis on RyR2. Ectopic activity was triggered by membrane depolarizations (delayed afterdepolarizations), primarily occurring in epicardium and were prevented by KN-93. The results reveal that arrhythmias after acidosis are dependent on CaMKII activation and are associated with an increase in SR Ca2+ load, which appears to be mainly due to the increase in PT-PLN.
Keywords: sarcoplasmic reticulum, calcium/calmodulin-dependent protein kinase
cardiac arrhythmias are a leading cause of morbidity and mortality. Despite their importance, a clear comprehension of the mechanisms underlying life-threatening ventricular tachyarrhythmias is lacking (6, 23). Different types of evidence indicate that acidosis is able to generate arrhythmias in the heart (27, 37). This is important in the clinical setting since substantial changes in extracellular and/or intracellular pH may occur in several disorders of different origin, like sleep apnea/hypopnea syndrome, diabetic ketoacidosis, or in patients on dialysis, which affect cardiac function (32). Moreover, a marked acidosis occurs during myocardial ischemia, which may play a crucial role in the arrhythmogenesis typical of ischemia-reperfusion injury (6). Although it is known that acidosis may produce arrhythmias by its actions either at the single myocyte level or in the conduction pathways within a multicellular preparation, the molecular mechanism of these arrhythmias remains elusive (6). At the single cell level, arrhythmias may be produced by changes in automaticity or they can be triggered either by early afterdepolarizations (EADs) or delayed afterdepolarizations (DADs). EADs are membrane depolarizations that appeared before the completion of the action potential (AP). It is generally accepted that they arise from current flowing through L-type Ca2+ channels (2). In contrast to EADs, DADs occur following the repolarization of the AP and have been associated to the higher frequency of sarcoplasmic reticulum (SR) Ca2+ sparks produced by a Ca2+-overloaded SR. This results in a Ca2+-activated transient-inward current, which has been mainly related to the current produced by the electrogenic Na+-Ca2+ exchanger (NCX) working in the forward mode (41). Whereas EADs have been associated to the activity of Ca2+/calmodulin-dependent protein kinase (CaMKII) (2), this association is not clear for DADs.
Different laboratories, including our own, have shown that the mechanical recovery after an acid load is primarily dependent on CaMKII activity (14, 34, 36). In particular, the CaMKII-dependent phosphorylation of Thr17 of phospholamban (PLN), the main regulatory protein of sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), appears to be important to offset the direct inhibitory effect of acidosis on SERCA2a and therefore of the recovery of relaxation and SR Ca2+ content during acidosis (14, 31, 34). During the course of these experiments in perfused rat hearts, we observed arrhythmic contractions that appeared after ∼15 min of acidosis in a few preparations but were evident in all preparations upon returning to normal pH. A similar pattern was described in isolated myocytes (36). Interestingly, the onset and removal of the acid stimulus have been associated to the spontaneous SR Ca2+ release in both nonstimulated and electrical-stimulated preparations (37). From these results it is reasonable to expect that the arrhythmias observed during acidosis and postacidosis are primarily triggered by a Ca2+-overloaded SR due to CaMKII activation and PLN phosphorylation. The present experiments were undertaken to test these hypotheses.
METHODS
Animals
Experiments were performed in Wistar male rats (200–300 g body wt) and in transgenic mice (25–30 g body wt) expressing four concatenated repeats of the CaMKII autocamitide inhibitory peptide (AIP) selectively in the SR membrane (SR-AIP). Age-matched wild-type (WT) mice served as controls. The mouse transgenic model was developed as previously described (21). Animals used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH) Publication No. 85-23, Revised 1996]. The protocol was approved by the Ethic Committee of the Cardiovascular Research Center, National Research Council (CCT-La Plata Consejo Nacional de Investigaciones Científicas y Tecnológicas, Argentina).
Intact Hearts
Heart perfusions.
Isolated hearts were perfused according to Langendorff technique at constant temperature (37°C) and flow (14 and 4 ml/min for rat and mouse hearts, respectively). After ablation of the atrioventricular (AV) node, the heart rate was kept at 240 and 360 beats/min for rat and mouse hearts, respectively, unless otherwise stated. The physiological bicarbonate buffer solution (BBS) contained (in mM) 128.3 NaCl, 4.7 KCl, 1.35 CaCl2 (2.5 in mice), 20.2 NaHCO3, 0.4 NaH2PO4, 1.1 MgCl2, 11.1 glucose, and 0.04 Na2EDTA; this solution was equilibrated with 95% O2-5% CO2 to give a pH of 7.4 (control solution). Mechanical parameters were obtained by passing into the left ventricle a latex balloon connected to a pressure transducer. The balloon was filled with aqueous solution to achieve a left ventricular end-diastolic pressure of 6–12 mmHg (34). Monophasic APs (MAPs) were obtained by using a Ag/AgCl electrode apposed to the epicardial free left ventricular wall, using a direct current—coupled high—input impedance differential amplifier. The MAP electrode was gradually positioned with the help of a micromanipulator until a gentle but stable contact pressure was achieved (4, 25). Recordings were accepted for analysis if they had a stable baseline, a rapid upstroke with consistent amplitude, and a smooth contoured repolarization phase and if they remained stable throughout the stabilization period. In most of the experimental series, MAPs were recorded both in the absence and presence of a latex balloon, to allow for simultaneous mechanical measurements in the latter case.
Experimental protocol.
After stabilization (control solution, pH 7.4), hearts were perfused with BBS equilibrated with 80% O2-20% CO2 (hypercapnic acidosis, pH 6.8) for 20 min and then returned to the control solution. Quantification of ectopic activity was accomplished by counting the number of beats occurring between triggered electrical activity during a period of 3 min (see results). A group of hearts was freeze clamped at different times during acidosis and at 1 and 3 min after the acidosis period, the time at which arrhythmias were more profuse, for biochemical assays. When drugs were used, they were perfused 10 min before the beginning of acidosis and throughout the acidosis and postacidosis periods, unless otherwise indicated. The concentration of DMSO used for the dilution of the drugs failed to affect the basal contractility and the pattern of ectopic activity. In some experiments, a defined pacing protocol with pauses test for spontaneous activity was performed to detect the possible appearance of DADs. After the usual protocol of stabilization and acidosis at 240 beats/min, the stimulation frequency was stopped to allow for spontaneous activity at the moment of returning to normal pH. The usual spontaneous rhythm of the hearts after AV node blockade was 70–80 beats/min. In some of these experiments, two electrodes were simultaneously apposed to the endocardial (septum) and the epicardial surface of the left ventricle.
SR membrane vesicles.
SR membrane vesicles were prepared as previously described (16). Protein was measured by the method of Bradford using bovine serum albumin as the standard. The yield was 1 to 2 mg membrane vesicles protein/g tissue.
Electrophoresis and western blot analysis.
For immunological detection of PLN and phosphorylated PLN, 15 μg of membrane protein were electrophoresed per gel lane in 10% acrylamide gels (34). For immunological detection of ryanodine receptor type 2 (RyR2) and phosphorylated RyR2, 50 μg of membrane protein were electrophoresed per gel lane in 6% acrylamide gels (16). Separated proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore) and probed with the following antibodies: phospho- (p)Ser16-PLN (1:5,000), pThr17-PLN (1:5,000), and RyR2-pS2809 (1:5,000; Badrilla, Leeds, UK), and RyR2-pS2815 (1:1,000; kindly provided by X. Wehrens, Houston, TX), and RyR2 (1:2,500; Affinity Bioreagents). Immunoreactivity was visualized by peroxidase-conjugated antibodies using a peroxidase-based enhanced chemiluminescence detection kit (ECL, Amersham). The signal intensity of the bands was quantified using ImageJ (NIH). Phosphorylation of PLN was expressed as the percentage of the control values (previous to acidosis) and the absence of drugs. RyR2 phosphorylation was normalized by the total RyR2 content and expressed as the percentage of control.
Isolated Myocytes
Myocyte isolation.
Rat myocytes were isolated by enzymatic digestion (38) and kept in a HEPES-buffered solution at room temperature (20–22°C) until used. Only rod-shaped myocytes with clear and distinct striations and an obvious marked shortening and relaxation on stimulation were used. Experiments were performed at room temperature.
Indo-1 fluorescence and cell shortening measurements.
Myocytes were loaded with indo-1 AM (17 μM for 9 min) (38). Cells were placed on the stage of an inverted microscope (Nikon Diaphot 200) adapted for epifluorescence, continuously superfused with BBS (pH 7.4) at a constant flow of 1 ml/min, and field stimulated via two platinum electrodes on either side of the bath at 0.5 Hz. The ratio of the indo-1 emission (410 and 490 nm) was taken as an index of intracellular Ca2+. Resting cell length and cell shortening were measured by a video-based motion detector (Crescent electronics, UT). Indo-1-loaded myocytes were subjected to the protocol of hypercapnic acidosis and then returned to control pH, as described above. SR Ca2+ content and SR Ca2+ leak were assessed at different times during this protocol (see results). SR Ca2+ content was determined by rapidly switching from the BBS to one of the same pH, containing 25 mM caffeine to cause SR Ca2+ release. SR Ca2+ leak was studied according to Shannon et al. (45). In short, the method consists in measuring resting Ca2+ in the presence and absence of SR Ca2+ channel blockade by tetracaine. At selected times during the protocol, the stimulation was stopped and the myocytes were exposed to 0 Na+-0 Ca2+ solution for 60 s to block the NCX, so that little or no Ca2+ can enter or leave the resting cell, in the absence and presence of tetracaine to block the SR Ca2+ release channel. The difference in diastolic Ca2+ with and without tetracaine was taken as an estimation of SR Ca2+ leak.
All data (perfused hearts and isolated myocytes) were recorded on a hard disk at a sampling frequency of 1 kHz by using PowerLab data acquisition software and a personal computer.
Planar Lipid Bilayers
Isolation of cardiac SR microsomes from rat ventricle and single RyR2 channel recordings were carried out as previously described (7, 11). Briefly, cardiac SR microsomes were fused to phosholipid planar bilayers (5 phosphatidylethanolamine:4 phosphatidylserine:1 phosphatidylcholine parts, 50 mg/ml in decane), painted on a 100-μm hole separating two compartments: CIS or cytosolic (containing 250 mM HEPES/Tris-OH, pH 7.9) and TRANS or lumenal [250 mM HEPES/52 mM Ca(OH)2, pH 7.4]. In all experiments, the membrane potential equaled 0 mV. RyR2 openings are observed as upward deflections of ∼3.5 pA (Ca2+ flux: TRANS→CIS). Recordings were filtered at 1 kHz, digitized at 5 kHz with a Digidata 1360 (Axon Instruments), and analyzed using pClamp9 and SigmaPlot 9 (Systat Software, San Jose, CA).
The cytosolic pH was decreased in steps (from 7.9 to 6.6) by a cumulative addition of HEPES in two sets of experiments: 1) with 2 μM cytosolic-free [Ca2+], where RyR2 are moderately active; and 2) with 200 μM [Ca2+], where RyR2 are fully activated (11). Since we used the fairly pH-insensitive Ca2+ chelator Dibromo-BAPTA, only a minor adjustment was required at the most acidic pH. At each pH step, 4-min recordings were taken to estimate the individual open probability (Po). To test the reversibility of the effect of acidity on RyR2 bathed with 2 μM cytosolic-free [Ca2+], pH was changed from 6.6 back to 7.3 by an addition of Tris-OH. To roughly estimate the rate of recovery, Po samples were taken every 10 s before and after changing the pH.
Statistics.
Data are expressed as means ± SE. Statistical significance was determined by Student's t-test for paired or unpaired observations as appropriate and by ANOVA when different groups were compared. The Newman-Keuls test was used to examine statistical differences observed with the ANOVA. A P value < 0.05 was considered statistically significant.
RESULTS
Arrhythmias After Acidosis
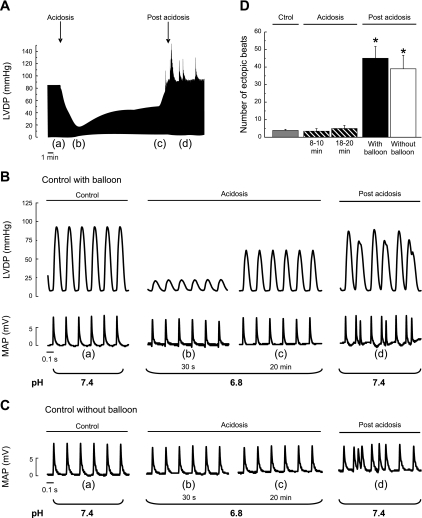
Figure 1A shows typical records of the time course of left ventricular developed pressure during acidosis and after returning to normal pH. As already described, hypercapnic acidosis produced an impairment of contractility followed by a spontaneous recovery that occurred despite the persistent extracellular acidosis. Returning to normal pH triggers an arrhythmic pattern. Figure 1B is an enlarged view of left ventricular developed pressure and epicardial MAPs at different times during the protocol (a–d in Fig. 1A). MAP recordings indicated that the ectopic beats occur after the completion of the paced beats. Similar results were obtained in the absence of the intraventricular balloon to avoid possible irritations (Fig. 1C). Figure 1D shows the overall results of these experiments.
Fig. 1.
Left ventricular developed pressure (LVDP) and epicardial monophasic action potentials (MAPs) during acidosis and postacidosis. A: representative mechanical recordings showing the time course of changes in LVDP during acidosis and upon returning to normal pH (postacidosis). B: enlarged records of LVDP and epicardial MAPs corresponding to points a to d of the record in A. The arrhythmic pattern that occurs when the heart was returned to normal pH is illustrated. C: records of MAPs obtained in the absence of intraventricular balloon. D: overall results of the experiments of this series. Each bar represents the mean occurrence of ectopic beats measured under each condition during a 3-min period: the last 3 min of the stabilization (C), the last 3 min of the first and second half of the 20 min of acidosis, and the first 3 min after returning to normal pH in the presence and absence of intraventricular balloon are shown. *P < 0.05 with respect to control (Ctrol).
In an additional group of experiments in which metabolic acidosis was elicited by decreasing the NaHCO3 concentration of the perfusate (pH 6.8), no mechanical recovery was observed and the ectopic beats after acidosis were significantly less than those with hypercapnic acidosis (results not shown).
Role of CaMKII
Perfused rat hearts.
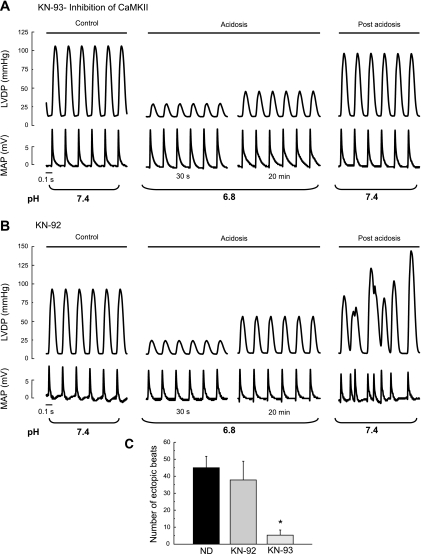
To investigate the hypothesis that arrhythmias are favored by CaMKII activation during acidosis, the same protocol showed in Fig. 1 was followed in the presence of 1 μM of the CaMKII inhibitor KN-93 and of the inactive analog, KN-92 (Fig. 2, A and B). Whereas the mechanical recovery and the arrhythmic activity were greatly reduced in the presence of KN-93, both persisted in the presence of KN-92. Overall results are depicted in Fig. 2C.
Fig. 2.
LVDP and epicardial MAPs during acidosis and postacidosis in the presence or in the absence of CaMKII inhibitor KN-93 and the inactive analog, KN-92. A: 1 μM KN-93 abolished the ectopic beats that appear immediately after returning to normal pH. B: lack of effect of KN-92 (1 μM) on the arrhythmias of postacidosis. C: overall results of this series. In this and the following figures, each bar represents the mean occurrence of ectopic beats measured during the first 3 min after returning to normal pH. ND, no drugs added. *P < 0.05 with respect to ND.
In additional experiments it was found that the arrhythmic pattern observed after acidosis persisted in the presence of 1 μM H-89 and 1 μM chelerythrine used to specifically inhibit protein kinase A and C, respectively (9, 19). The number of ectopic beats in a 3-min period after acidosis was 33 ± 1 and 30 ± 6, respectively (n = 3 in both cases).
Table 1 shows the lack of effect of the different kinase inhibitors on basal contractility, relaxation, and MAPs duration.
Table 1.
Effects of the different interventions on basal contractility, relaxation, and MAPDs
| n | LVDP, mmHg | +dP/dt, mmHg/s | t1/2, ms | MAPD90, ms | |
|---|---|---|---|---|---|
| Control | 101.9±4.8 | 2799.8±89.0 | 53.0±1.5 | 53.3±4.1 | |
| KN-93 (1 μM) | 3 | 93.2±6.7 | 2628.7±189.7 | 54.2±3.1 | 61.2±6.2 |
| Control | 103.5±5.7 | 2762.5±112.7 | 54.7±2.6 | 54.0±2.1 | |
| KN-92 (1 μM) | 4 | 99.2±5.4 | 2754.9±173.7 | 54.7±3.6 | 50.8±8.5 |
| Control | 110.9±11.8 | 3601.6±411.1 | 67.7±4.8 | 68.7±4.1 | |
| Ryanodine (30 nM) | 3 | 51.4±4.8* | 1600.2±169.8* | 82.3±6.2* | 66.7±2.9 |
| Control | 92.9±3.1 | 2744.0±270.6 | 55.2±1.7 | 61.3±6.3 | |
| Thapsigargin (1 μM) | 4 | 62.9±5.2* | 1411.3±143.7* | 67.5±2.6* | 58.7±4.2 |
| Control | ND | ND | ND | 50.5±4.0 | |
| Chelerythrine (1 μM) | 3 | ND | ND | ND | 55.0±2.9 |
| Control | ND | ND | ND | 46.8±6.3 | |
| H-89 (1 μM) | 3 | ND | ND | ND | 50.3±2.3 |
| Control | ND | ND | ND | 57.3±1.8 | |
| Dantrolene (1 μM) | 3 | ND | ND | ND | 53.0±4.3 |
| Wild-type mice | 3 | 80.3±7.6 | 3449.7±250.9 | 35.5±1.3 | 44.6±3.4 |
| SR-AIP mice | 3 | 75.7±6.7 | 2682.6±316.6 | 44.2±1.1* | 42.1±2.9 |
Values are means ± SE (n, number of experiments) and were obtained at the end of the stabilization (control) and at the end of the 10-min period of drug perfusion, previous to acidosis. LVDP, left ventricular developed pressure; +dP/dt, maximal rate of pressure development; t1/2, half relaxation time; MAPD90, monophasic action potential duration at 90% of repolarization. SR-AIP, sarcoplasmic reticulum-autocamitide inhibitory peptide; ND, no determination.
P < 0.05 vs. control.
Transgenic mice with targeted inhibition of CaMKII in cardiac SR membrane (SR-AIP).
Figure 3, A and B, shows that although the ectopic activity after acidosis and the mechanical recovery during acidosis were present in WT mice, both phenomena were significantly decreased in the SR-AIP mice. A similar arrhythmic pattern was observed when the balloon was omitted (not shown). Figure 3C shows the overall results of these experiments. Table 1 summarized the basal values of MAPs duration, contractility, and relaxation in WT and SR-AIP mice.
Fig. 3.
LVDP and epicardial MAPs during acidosis and postacidosis in wild-type (WT) and sarcoplasmic reticulum-autocamitide inhibitory peptide (SR-AIP) mice. A and B: records of LVDP and epicardial MAPs, corresponding to the control, acidosis, and postacidosis periods, in WT mice (A) and SR-AIP mice (B). In WT mice there was a mechanical recovery during acidosis, similar to rat hearts, followed by an arrhythmic pattern upon returning to normal pH. In the SR-AIP mice, both the mechanical recovery and the ectopic activity was significantly decreased. C: overall results of this experimental series. +dP/dt, maximal rate of pressure development. *P < 0.05 with respect to WT mice.
Arrhythmias upon Removal of the Acid Stimulus Are Dependent on the SR Ca2+ Load and Release
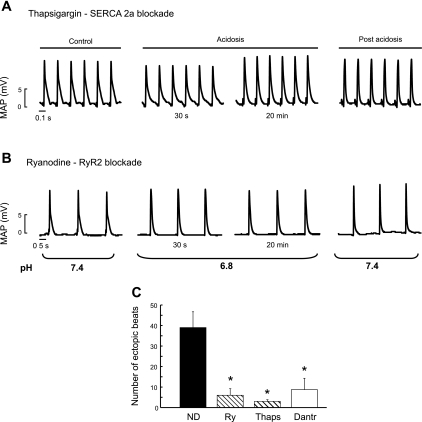
To study whether the arrhythmias observed depend on SR Ca2+ load and release, we used thapsigargin, to inhibit SR Ca2+ uptake and ryanodine or dantrolene, to inhibit SR Ca2+ release. Figure 4, A and B, depicts typical examples of the thapsigargin and ryanodine experiments. Both drugs produced a significant reduction in the occurrence of ectopic beats after removal of acidosis. Moreover, the mechanical recovery was virtually abolished in both cases (not shown). Similar results were obtained with dantrolene. The overall results of these experiments are shown in Fig. 4C.
Fig. 4.
Ectopic beats are prevented by inhibition of SR Ca2+ uptake and release. Traces showing that ectopic beats after returning to normal pH are prevented in the presence of inhibition of SR Ca2+ uptake (thapsigargin, 1 μM; A) or SR Ca2+ release (ryanodine, 30 nM; B). Note that stimulation was reduced in the ryanodine experiments to avoid an excessive increase in left ventricular end-diastolic pressure. C: overall results of this series. SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase 2a; RyR2, ryanodine (Ry) receptor type 2; Thaps, thapsigargin; Dantr, dantrolene. *P < 0.05 with respect to ND.
SR Ca2+ Load Is Associated with Thr17 Phosphorylation of PLN
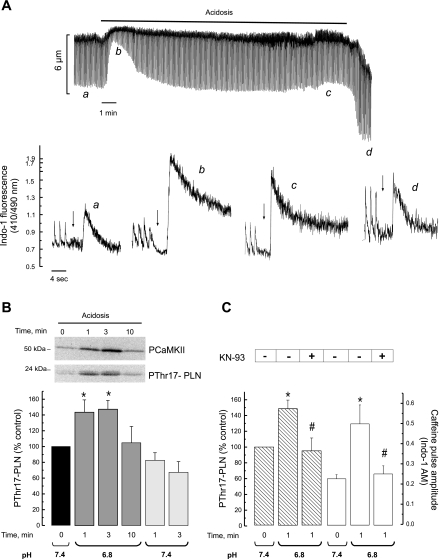
Figure 5A shows the time course of the effect of hypercapnic acidosis on cell shortening in isolated myocytes and typical recordings of the caffeine-induced Ca2+ transient, as an estimation of total SR Ca2+ content, before acidosis (a), at the beginning (b), and at the end (c) of acidosis and after returning to normal pH (d). SR Ca2+ content increases after 1 min of acidosis, the moment during acidosis at which the Thr17 phosphorylation of PLN was maximal (Fig. 5B). The SR Ca2+ content then diminished but was still significantly higher than control by the end of the acidosis period and after acidosis (See Fig. 6). The records in Fig. 5A also showed that acidosis significantly slowed the caffeine Ca2+ transient decay. The overall results indicated that time to 50% decay of the caffeine Ca2+ transient significantly increased from 387 ± 56 (control) to 939 ± 308 ms (acidosis, P < 0.05) and returned to control values, 350 ± 35 ms, 1 min after the acidosis period. In agreement with previous findings, these results reflect the acidosis-induced inhibition of the NCX (47) and further show that this inhibition was fully reversible immediately after returning to a normal pH. Figure 5B shows typical immunoblots of the phosphorylation of CaMKII and Thr17 of PLN. Both phosphorylations increased at the beginning of acidosis, returning to basal levels after ∼10 min of acidosis. Phosphorylation of Thr17 of PLN was also studied after acidosis. Although it showed a trend to decrease, this decrease did not attain significant levels. Phosphorylation of Ser16 of PLN, the PKA site, did not change (data not shown). Figure 5C shows that CaMKII inhibition decreased both, the significant increase in Thr17 phosphorylation at the beginning of acidosis and the associated increase in SR Ca2+ content. Altogether these results indicate that the CaMKII-dependent phosphorylation of Thr17 of PLN, although transient, is a major cause of the increase in SR Ca2+ load that occurs during acidosis, in line with previous findings from our and other laboratories (14, 31, 34, 36). Interestingly, additional experiments in perfused hearts in which KN-93 was administered during the acidosis period, immediately after the decay of Thr17 phosphorylation, failed to avoid the arrhythmic pattern: upon returning to control pH the number of ectopic beats recorded were 38 ± 7, a figure similar to that observed in the absence of drugs in the same time period (Fig. 1D).
Fig. 5.
CaMKII-inhibition prevented the acidosis-induced increase in phosphorylation (p) of Thr17 site of phospholamban (PLN) and in SR Ca2+ content. A: typical recordings of cell shortening (top) and intracellular Ca2+ obtained in isolated myocytes. The height of the caffeine-induced Ca2+ transient used to examine the SR Ca2+ content was greater at the beginning of acidosis and then slowly decreased during acidosis and in the postacidosis period. Notice that the rate of intracellular Ca2+ decay of caffeine pulses was reversible decreased during acidosis. The arrows indicate the moment at which caffeine was administered. B: inmunoblots and overall results of the phosphorylation of CaMKII before and during acidosis and Thr17 site of PLN before, during, and after acidosis (postacidosis). Bars represent overall results of phosphorylation of Thr17 of PLN. During acidosis, phosphorylation of CaMKII and Thr17 of PLN showed a similar pattern. Phosphorylation of Thr17 did not significantly change after acidosis. Of note, maximal phosphorylation of Thr17 occurs in association with maximal increase in SR Ca2+ content during acidosis (A). C: the increase in the phosphorylation of Thr17 of PLN and SR Ca2+ content at the beginning of acidosis were both significantly diminished by pretreatment with 1 μM KN-93 (n = 3–6 experiments). *P < 0.05 with respect to control; #P < 0.05 with respect to acidosis without KN-93.
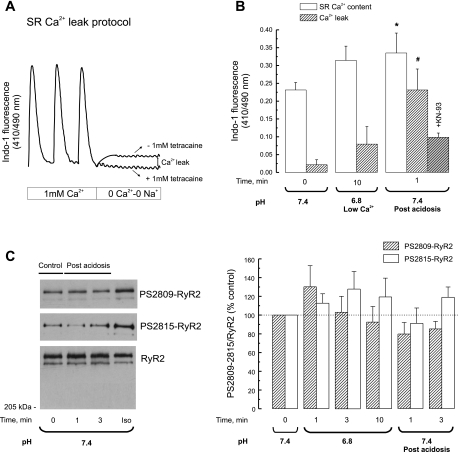
Fig. 6.
CaMKII-inhibition prevented the SR Ca2+ leak observed upon returning to normal pH. A: scheme of the protocol used to assess SR Ca2+ leak. In indo-1-loaded myocytes, stimulation was stopped and the myocytes were exposed to 0 Na+-0 Ca2+ solution for 60 s to block the Na+-Ca2+ exchanger (NCX) so that little or no Ca2+ can entered or left the resting cell in the absence and presence of tetracaine to block the SR Ca2+ release channel. The difference in diastolic Ca2+ with and without tetracaine was taken as an estimation of SR Ca2+ leak. SR Ca2+ leak was measured in the presence and absence of 1 μM KN-93. B: comparison of the average SR Ca2+ load and leak at pH 7.4 (control) at the end of acidosis and at the postacidosis period. The return to normal pH after acidosis increased SR Ca2+ leak with respect to control myocytes with a lower SR Ca2+ load and to myocytes submitted to acidosis with a similar SR Ca2+ load achieved by decreasing extracellular Ca2+. In the presence of KN-93, SR Ca2+ leak after acidosis was not significantly different from control (n = 3 experiments in each experimental group). C, left: immunoblots showing the phosphorylation of Ser2815 and Ser2809 of RyR2 before acidosis and upon returning to normal pH. Iso, phosphorylation of both RyR2 residues observed in hearts perfused with isoproterenol as a positive control. C, right: overall results of these experiments (n = 3 to 4 experiments). No significant alterations in the phosphorylation of RyR2 were detected. *P < 0.05 vs. control; #P < 0.05 vs. acidosis.
The Return to Normal pH Increased SR Ca2+ Leak and Relieves the RyR2
To examine whether the return to normal pH produces a leak of Ca2+ from an overloaded SR, the SR Ca2+ leak was studied in isolated myocytes after the acidosis period. A second group of myocytes was studied under control conditions (myocytes not submitted to acidosis). This group was paced at the same rate as the group submitted to acidosis and for the same period as the acidotic period. A third group of myocytes was studied at the end of acidosis. In this group the extracellular Ca2+ was decreased to 0.5 mM to match a similar SR Ca2+ content as the one observed after acidosis. Figure 6A depicts the protocol used. Figure 6B shows the overall results of these experiments. The return to normal pH was associated with an increase in SR Ca2+ leak when compared with control (same pH and lower SR Ca2+ content) or with the end of acidosis period (lower pH and similar SR Ca2+ content). As expected, the increase in SR Ca2+ leak observed after acidosis significantly decreased toward control values when the acidosis period was induced in the presence of KN-93. Taken together, these results would indicate that the return to normal pH evokes the leak of Ca2+ from a Ca2+-overloaded SR. An increase in spontaneous SR Ca2+ release has been previously related to either PKA or CaMKII-dependent phosphorylation of RyR2 at either Ser2809 or Ser2815 sites, respectively (12, 30). Figure 6C shows Western blots and the overall results indicating that no significant changes were observed in the phosphorylation of Ser2809 or Ser2815 of RyR2 either during acidosis or after returning to normal pH with respect to control values. Thus the increase in SR Ca2+ leak observed after returning to normal pH with respect to that observed during acidosis for a similar SR Ca2+ load may be attributed to a reversible inhibition of RyR2 by acidosis.
Acidosis Reversibly Inhibits RyR2
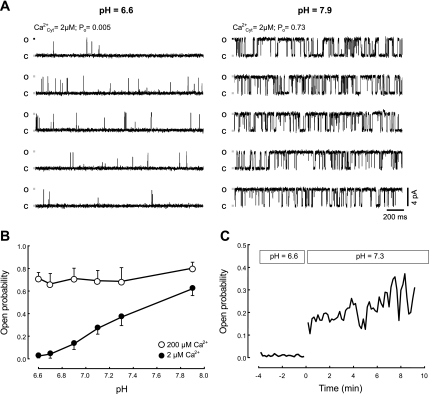
Although it is generally held that acidosis inhibits RyR2, there are some differences between reports testing the action of protons on RyR2 (44, 51). On the one hand, increasing/decreasing the pH around physiological levels (pH ∼7.2), respectively, activated/inhibited RyR2 (44). In another report, only the inhibition was found at pH < 7 (51). These discrepancies may reflect differences in experimental conditions, including levels of cytosolic free [Ca2+] (51). In Fig. 7, we then compared the effect of changing pH at the cytosolic surface of RyR2 bathed with 2 μM free cytosolic [Ca2+] (∼EC50 for RyR2 activation) or with 200 μM (which fully activates the channels) (11).
Fig. 7.
Cytosolic pH affects RyR2 activity at low (2 μM) cytosolic-free Ca2+. A: single channel recordings at pH 7.9 (right) and 6.6 (left). Recording conditions were high-luminal [Ca2+] (50 mM), membrane potential = 0 mV, and 2 μM cytosolic-free [Ca2+]. B: mean open probability (Po) as a function of pH observed in RyR2 bathed with 2 μM cytosolic free [Ca2+] (•, n = 6 experiments) or with 200 μM (○, n = 5 experiments). C: mean Po of RyR2 channels as a function of time (t) (n = 4 experiments). At pH = 6.6 (t < 0), RyR2 remained closed. At t = 0, pH was changed back to 7.3 (for details, see methods). Ca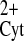 , cytosolic Ca2+; O, open; C, closed.
, cytosolic Ca2+; O, open; C, closed.
Figure 7, A and B, shows that with cytosolic-free [Ca2+] (∼2 μM), Po decreased at each step of acidification. The Hill equation, Po = Po0/{1 + ([H+]/IC50)N} was fitted to these data. Po0 (Po predicted in the absence of H+) was 0.65 ± 0.04, IC50 ([H+] that induces half-maximal inhibition) was 63 ± 7 nM (equivalent to pH ∼7.2), and N (minimum apparent number of H+ inhibitory sites in RyR2) was 2.0 ± 0. The inhibitory action of protons on RyR2 was readily reversed (within ∼30 s) by increasing the pH from 6.6 to 7.3 (Fig. 7C). When the RyR2 were bathed with saturating cytosolic-free [Ca2+], Po values did not decrease with acidity (Fig. 7B, open circles). The results suggest that protons decreased the affinity of the RyR2 activating cytosolic sites for Ca2+.
CaMKII-Dependent DADs Can Be Detected After the Acidosis Period
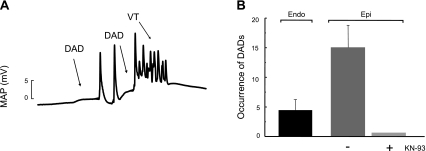
The dependence of the arrhythmic pattern described on the SR Ca2+ release and SR Ca2+ content would suggest that arrhythmias may be triggered by DADs. We therefore followed a defined pacing protocol with pauses test for spontaneous activity to detect the possible appearance of DADs (see methods). Figure 8A shows MAPs recorded from the epicardial wall after this protocol. After termination of pacing at 240 beats/min and simultaneous return to normal pH, two membrane depolarizations were observed. Whereas the first was followed by two spontaneous beats, the second triggered an episode of ventricular arrhythmia. This behavior was observed in five of five experiments of this type and in zero of three experiments when KN-93 was present (Fig. 8B). When MAPs were recorded simultaneously from the endocardial and epicardial ventricular wall, the number of DADs detected from the endocardial wall was significantly lower than that detected from the epicardial surface (4 ± 2 vs. 15 ± 4, n = 5) in the three first minutes of normal pH after acidosis. Taken together, these experiments would indicate that the returning to normal pH evoked membrane depolarizations, suggestive of DADs, mainly detected from the epicardial surface of the ventricular wall, which are able to trigger arrhythmic episodes and are prevented by CaMKII inhibition.
Fig. 8.
Arrhythmias after acidosis are triggered by membrane depolarizations typical of delayed afterdepolarizatons (DADs). A: protocol of pacing and pauses tests for spontaneous activity. Hearts were paced at 240 beats/min during acidosis and then returned to normal pH in the absence of pacing to allow for spontaneous activity. The trace shows that after acidosis, spontaneous membrane depolarizations typical of DADs triggered the ectopic activity. B: overall results showing that DADs developed mainly in the epicardial (Epi) surface and are prevented by pretreatment with KN-93. VT, ventricular tachycardia; Endo, endocardial.
DISCUSSION
In the present study we investigated the cellular mechanisms underlying the arrhythmias that occur after acidosis, upon returning to normal pH. We observed that arrhythmias are dependent on the activity of the SR. More importantly, we showed that the ectopic activity is suppressed by the inhibition of the multifunctional Ca2+/calmodulin-dependent protein kinase, CaMKII, and did not occur in a transgenic mouse model with the inhibition of CaMKII targeted to the SR. Arrhythmias appear to be triggered by slow membrane depolarizations, typical of DADs, that were also prevented by CaMKII inhibition. The inhibition of CaMKII also blunted the increase in phosphorylation of Thr17 of PLN and in SR Ca2+ content that occurred associated with the onset of acidosis, as well as the increased SR Ca2+ leak that occurred after returning to a normal pH. The results further showed that the return to a normal pH reversed the acidosis-induced inhibition of RyR2 activity and of the NCX. Taken together, these findings indicate a primary role of CaMKII on the enhancement of the SR Ca2+ content observed during acidosis and suggest that the phosphorylation of Thr17 of PLN may be possibly involved in this effect, in agreement with previous findings (14, 34, 36). The results further suggest that the spontaneous activity that takes place after returning to normal pH is triggered by CaMKII-dependent DADs, which would be favored by two concurrent factors: 1) the CaMKII-dependent enhancement of the SR Ca2+ content that occurred at the beginning of acidosis and still persists after acidosis and 2) the simultaneous relief of RyR2 and NCX previously inhibited by acidosis.
Monophasic APs
MAPs are extracellularly recorded waveforms that are not identical to true transmembrane AP recordings. Nevertheless, they can accurately reproduce the time course of transmembrane APs and are suitable for studying the characteristics of local myocardial electrophysiology in intact animal preparations and in the clinical setting (5, 8, 13, 17, 33). Indeed, recording MAPs is the only possible method to explore localized myocardial activation and repolarization in the human heart or in the in vivo animal hearts. In the present experiments we used MAP recordings to assess the type of arrhythmia that takes place upon returning to a normal pH after acidosis. Although the application of this method has several practical problems such as MAPs instability or registration of artifacts, we were able to minimize the influence of these problems and to obtain stable MAP recordings that could be simultaneously assessed in the endocardial and epicardial ventricular walls and could be suppressed by different interventions.
Arrhythmias After Acidosis Are Suppressed by CaMKII Inhibition
The present results showed that the ectopic activity after acidosis was significantly decreased in transgenic animals with the inhibition of CaMKII targeted to the SR, compared with that in the age-matched WT mice. The arrhythmic pattern was also prevented by the CaMKII inhibitor, KN-93, but not by its inactive analog KN-92, in the perfused rat heart. The arrhythmias appeared to be triggered by membrane depolarizations, typical of DADs that were also blocked by KN-93. These results indicate that the triggered arrhythmias are dependent on a CaMKII phosphorylation, which would likely occur at the SR level. Since RyR2 were not significantly phosphorylated either during or after acidosis, these experiments support the notion of a major role of PLN phosphorylation, specifically the CaMKII site Thr17, in the increase in SR Ca2+ content that occurs at the beginning of acidosis. Recent evidence indicated that SR-AIP mice show a consistent decrease in CaMKII-dependent facilitation at the L-type Ca2+ channels level (40). However, acidosis either decreases or does not change the L-type Ca2+ current (20, 26), making a possible contribution of this current to the acidosis-induced increase in SR Ca2+ load unlikely. Moreover, if this contribution takes place, it would not fade the importance of Thr17 phosphorylation on this effect.
It has been previously shown that CaMKII inhibition reduces Ca2+ current facilitation, L-type Ca2+ channel opening probability, and EADs (2, 15, 49, 50). Taken together, these findings convincingly showed the link between EADs initiation, L-type Ca2+ current, and CaMKII activation. Moreover, a different type of evidence demonstrated that CaMKII may affect Na+ and K+ channels, which would be expected to modify AP duration (43, 48). We did not observe changes in AP duration after KN-93 administration (Table 1), which would preclude an effect of either the drug or of CaMKII on these channels under basal conditions.
DADs are not linked to ion-channel alterations but rather to conditions that favor SR Ca2+ overload (29). Earlier studies by Wu et al. (49) indicated that when artificially prolonged AP waves are used as voltage-clamp commands, cell membranes exhibit a transient-inward current that is blocked by the dialysis of a CaMKII inhibitory peptide. These results may suggest that Ca2+ influx during a prolonged AP is capable of overloading the SR with Ca2+, producing currents likely to be responsible for DADs (1). However, the potential role of CaMKII activity in DADs formation had never been directly tested. The present results indicate that the arrhythmias observed after acidosis are suppressed by CaMKII-inhibition and are likely triggered by DADs. In our experimental conditions, DADs primarily originated in the epicardium. This mechanism would constitute the main mechanism of triggered arrhythmias after a period of acidosis. In the context of these results, it is important to mention that although it has been previously thought that DADs originate mainly in the endocardium (28), recent work reported that DADs and the triggered activity associated with spontaneous SR Ca2+ release occurred preferentially near the epicardium in a model of abnormal RyR2 function induced by FKBP12.6 dissociation and β-adrenergic stimulation. The authors attributed the higher ectopy of the epicardium with respect to the endocardium to the faster SR Ca2+ uptake observed, possibly due to a higher expression of SERCA2a compared with that observed in the endocardium (22, 35).
Mechanism of SR Ca2+ Load During Acidosis
Previous experiments causally linked the spontaneous mechanical recovery that occurs during acidosis to an increase in SR Ca2+ load (18). Earlier evidence indicated that the signaling cascade involved in this increase was triggered by the activity of the Na+-H+ exchanger, enhanced by intracellular acidosis, which in turn would increase intracellular Na+ and intracellular Ca2+ by slowing the forward mode of the NCX and eventually reversing it. It was hypothesized that the increase in Ca2+ promoted by this pathway would be sufficient to overcome the direct inhibitory action of acidosis on the activity of SERCA2a (18, 39). More recent studies indicated, however, that this cascade of events, although possibly necessary, was not sufficient by itself to increase the Ca2+ content of the SR. It was shown that the activation of CaMKII and the phosphorylation of Thr17 of PLN were necessary events underlying the mechanical recovery (14, 34, 36). In line with these previous findings, the present experiments also show that acidosis produces an increase in SR Ca2+ load and a mechanical recovery, both of which were reduced by CaMKII inhibition, as it was the significant increase in the phosphorylation of Thr17 of PLN. The increase in the phosphorylation of Thr17 occurred at the beginning of acidosis associated to the maximal increase in SR Ca2+ content (Fig. 5). We are aware of the fact that the increase in SR Ca2+ content observed during acidosis is qualitative and may be distorted due to possible changes in cytoplasmic Ca2+ buffering. However, the increase in SR Ca2+ content during acidosis has been validated by quantitative measurements of SR Ca2+ and Ca2+ buffering and from changes in NCX current on repolarization (10). The present findings add to these previous results the fact that the increase in SR Ca2+ content during acidosis is dependent of CaMKII, in agreement with previous findings in mice by DeSantiago et al. (14). Taken together, the results suggest that the activation of CaMKII seems to be a necessary step required to increase SR Ca2+ load, possibly through the phosphorylation of Thr17 of PLN (14, 34). In this scenario and relevant to the present study, it is important to rescue the role of the acidosis-induced inhibition of RyR2 and NCX in favoring SR Ca2+ overload under acidosis conditions (see below).
Why Is the Ectopic Activity More Closely Associated to the Return to Normal pH?
If the increase in SR Ca2+ content occurs during acidosis, the question is, then, Why are the arrhythmias more closely associated to the return to normal pH? The explanation to this finding may be given by experimental evidence showing the inhibitory effects of acidosis on RyR2 and on the frequency of Ca2+ spark (3, 10, 44, 46, 51). In agreement with these results, we showed that acidosis reversibly inhibits the RyR2 open probability in planar lipid bilayers at not saturating cytosolic Ca2+ levels (see Fig. 7). Moreover, the SR Ca2 leak observed after returning to normal pH was not only greater than control, possibly due to a significant increase in SR Ca2+ content, but also more important than the Ca2 leak observed at the end of acidosis, for a similar SR Ca2+ load. An increase in SR Ca2+ leak may also be favored by a CaMKII-dependent phosphorylation of RyR2 (12, 30). However, our results did not detect any significant increase in the phosphorylation of either Ser2815 or Ser2809 sites, neither during the acidosis period nor after returning to normal pH. Thus returning to normal pH would increase the opening probability of the Ca2+ release channel of a Ca2+ overloaded SR. Returning to normal pH would also favor the reactivation of NCX inhibited by acidosis. In agreement with previous findings (47), the present experiments indeed showed that the rate of Ca2+ decline of caffeine transients was significantly slowed by acidosis, an effect that was fully reversible upon returning to normal pH. All these mechanisms, acting in concert, would be responsible for the increase in SR Ca2+ leak and triggered arrhythmias observed.
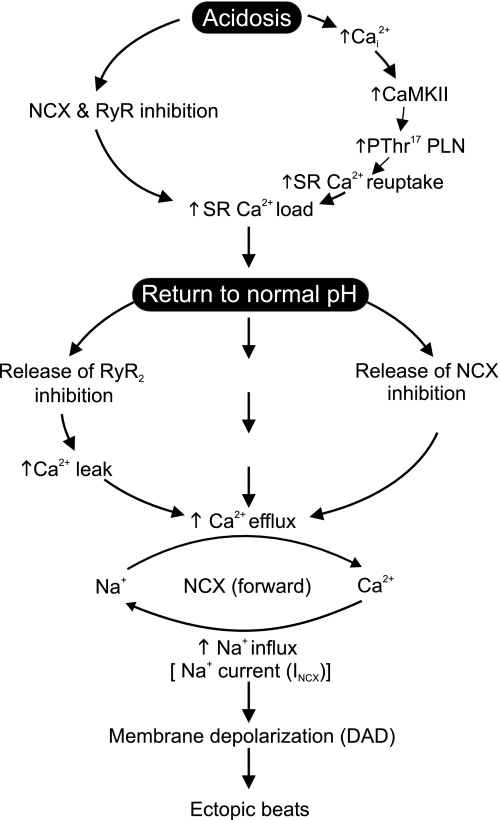
Figure 9 depicts the proposed mechanism for the arrhythmias triggered after a period of acidosis.
Fig. 9.
Cascade of events proposed to explain the arrhythmias triggered after acidosis upon returning to normal pH. The increase in the phosphorylation of Thr17 by acidosis-induced CaMKII activation, favored by the acidosis-induced inhibition of NCX and RyR2, would increase SR Ca2+ load. Alleviation of the acidosis-induced RyR2 and NCX inhibition, upon returning to normal pH, would produce an enhanced SR Ca2+ leak and Ca2+ efflux through the forward NCX mode, which would in turn increase Na+ influx. The increase in Na+ influx would underlie the depolarizing current that produces DADs. Ca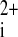 , intracellular Ca2+; INCX, current produced by NCX.
, intracellular Ca2+; INCX, current produced by NCX.
Clinical Implications
The present experiments indicated that the return to normal pH after a period of hypercapnic acidosis triggered an arrhythmic pattern that is dependent on CaMKII. Intracellular acidosis seems to be the important change, since metabolic acidosis produced a significant lower number of ectopic beats after returning to normal pH. Our findings may be of interest in the clinical setting, since substantial changes in intracellular pH may occur in different clinical disturbances of the acid /base status, like ischemia-reperfusion injury, the syndrome of sleep apnea/hypopnea (32), or in patients in dialysis (42), that may affect cardiac function. The present experiments suggest that alterations in intracellular pH associated with all these pathologies may be the substrate of at least part of the arrhythmias observed in these diseases.
CaMKII has emerged as an important arrhythmogenic signaling molecule in the setting of the LQT syndrome (49), cardiac hypertrophy (50), and cardiomyopathy (24). All these studies pointed to the crucial role of CaMKII in generating EADs and triggered arrhythmias. The present results strongly suggest that CaMKII is also responsible for DADs that trigger post-acidosis arrhythmias. As such, CaMKII may be an antiarrhythmic drug target during this type of arrhythmias.
GRANTS
This work was supported by Proyecto de Investigación Científica y Tecnológica Grants 14219 [Fondo para la Investigación Científica y Tecnológica, Argentina] (to M. Said) and 26117; Proyecto de Investgación Plurianual Grant 5300 (CONICET, Argentina); National Institutes of Health (NIH) Fogarty International Research Award Grant R03-TW-07713 (to A. Mattiazzi) and NIH Grant R01-GM-078665 (to J. A. Copello and P. L. Diaz-Sylvester).
Acknowledgments
The assistance of Inés Vera in making the illustrations is greatly acknowledged. We also thank Ariel Escobar and Daniel Lorenti for helpful comments and assistance in constructing the set up and electrodes for MAPs acquisition and to the student Nicolás Cédola for assistance in the last sets of experiments.
R. Becerra is a fellow from the Comisión de Investigaciones Científicas (Provincia de Buenos Aires, Argentina). M. Said, J. Palomeque, G. Rinaldi, C. Mundiña-Weilenmann, L. Vittone, and A. Mattiazzi are established Investigators of Consejo Nacional de Investgaciones de Científica y Technológica (CONICET, Argentina).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson ME Calmodulin kinase signalling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther 106: 39–55, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther 287: 996–1006, 1998. [PubMed] [Google Scholar]
- 3.Balnave CD, Vaughan-Jones RD. Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes. J Physiol 528: 25–37, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethell HW, Vanderberg JI, Smith GA, Grace AA. Changes in ventricular repolarization during acidosis and low-flow ischemia. Am J Physiol Heart Circ Physiol 275: H551–H561, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol 583: 695–704, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet E Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79: 917–1017, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain BK, Levitsky DO, Fleischer S. Isolation and characterization of canine cardiac sarcoplasmic reticulum with improved Ca2+ transport properties. J Biol Chem 258: 6602–6609, 1983. [PubMed] [Google Scholar]
- 8.Chen SA, Chiang CE, Yang CJ, Cheng CC, Wu TJ, Wang SP, Chiang BN, Chang MS. Sustained atrial tachycardia in adult patients. Electrophysiological characteristics, pharmacological response, possible mechanisms, and effects of radiofrequency ablation. Circulation 90: 1262–1278, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990. [PubMed] [Google Scholar]
- 10.Choi HS, Trafford AW, Orchard CH, Eisner DA. The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes. J Physiol 529: 661–668, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copello JA, Barg S, Onoue H, Fleischer S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys J 73: 141–156, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR. β-Adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100: 391–398, 2007. [DOI] [PubMed] [Google Scholar]
- 13.De Groot SH, Vos MA, Gorgels AP, Leunissen JD, van der Steld BJ, Wellens HJ. Combining monophasic action potential recordings with pacing to demonstrate delayed afterdepolarizations and triggered arrhythmias in the intact heart. Value of diastolic slope. Circulation 92: 2697–2704, 1995. [DOI] [PubMed] [Google Scholar]
- 14.DeSantiago J, Maier LS, Bers DM. Phospholamban is required for CaMKII-dependent recovery of Ca transients and SR Ca reuptake during acidosis in cardiac myocytes. J Mol Cell Cardiol 36: 67–74, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol 2: 173–177, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero P, Said M, Sánchez G, Vittone L, Valverde C, Donoso P, Mattiazzi A, Mundiña-Weilenmann C. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during β-adrenergic stimulation. J Mol Cell Cardiol 43: 281–291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franz MR Current status of monophasic action potential recordings: theories, measurements and interpretations. Cardiovasc Res 41: 25–40, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol Cell Physiol 262: C348–C357, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Herbert CJM, Augereaub JM, Gleyec J, Maffranda JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 172: 993–999, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Irisawa H, Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res 59: 348–355, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR. Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J Biol Chem 278: 25063–25071, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Katra RP, Oya T, Hoeker GS, Laurita KR. Ryanodine receptor dysfunction and triggered activity in the heart. Am J Physiol Heart Circ Physiol 292: H2144–H2151, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104: 569–580, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Khoo MS, Kannankeril PJ, Li J, Zhang R, Kupershmidt S, Zhang W, Atkinson JB, Colbran RJ, Roden DM, Anderson ME. Calmodulin kinase II activity is required for normal atrioventricular nodal conduction. Heart Rhythm 2: 634–640, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol 12: 1286–1294, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Komukai K, Pascarel C, Orchard CH. Compensatory role of CaMKII on ICa and SR function during acidosis in rat ventricular myocytes. Pflügers Arch 442: 353–361, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Kurachi Y The effects of intracellular protons on the electrical activity of single ventricular cells. Pflügers Arch 394: 264–270, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Laurita KR, Katra RP. Delayed after depolarization-mediated triggered activity associated with slow calcium sequestration near the endocardium. J Cardiovasc Electrophysiol 16: 418–424, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lederer WJ, Tsien RW. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol 263: 73–100, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Mattiazzi A, Vittone L, Mundiña-Weilenmann C. Ca2+/calmodulin-dependent protein kinase: a key component in the contractile recovery from acidosis. Cardiovasc Res 73: 648–656, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 173: 910–916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore HJ, Franz MR. Monophasic action potential recordings in humans. J Cardiovasc Electrophysiol 18: 787–790, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mundiña-Weilenmann C, Said M, Ferrero P, Vittone L, Kranias E, Mattiazzi A. Role of phosphorylation of Thr17 of phospholamban in the mechanical recovery from hypercapnic acidosis. Cardiovasc Res 66: 114–122, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Nam GB, Burashnikov A, Antzalevich Ch. Cellular mechanisms underlying the development of catecholaminergic ventricular tachycardia. Circulation 111: 2727–2733, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomura N, Satoh H, Terada H, Matsunaga M, Watanabe H, Hayashi H. CaMKII-dependent reactivation of SR Ca2+ uptake and contractile recovery during intracellular acidosis. Am J Physiol Heart Circ Physiol 283: H193–H203, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Orchard CH, Houser SR, Kort AA, Bahinski AA, Capogrossi MC, Lakatta EG. Acidosis facilitates spontaneous sarcoplasmic reticulum Ca2+ release in rat myocardium. J Gen Physiol 90: 145–165, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palomeque J, Sapia L, Hajjar R, Mattiazzi A, Vila-Petroff M. Angiotensin-II induced negative inotropy in rat ventricular myocytes: role of reactive oxygen species and p38 MAPK. Am J Physiol Heart Circ Physiol 290: H96–H106, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Pérez NG, Mattiazzi AR, Camilión de Hurtado MC, Cingolani HE. Myocardial contractility recovery during hypercapnic acidosis: its dissociation from recovery in pHi by ryanodine. Can J Cardiol 11: 553–560, 1995. [PubMed] [Google Scholar]
- 40.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol 42: 196–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1095–1096, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Redaelli B Hydroelectrolytic equilibrium change in dialysis. J Nephrol 14, Suppl 4: S7–S11, 2001. [PubMed] [Google Scholar]
- 43.Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/ calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther 317: 292–299, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Rousseau E, Pinkos J. pH modulates conducting and gating behaviour of single calcium release channels. Pflügers Arch 415: 645–647, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Spacapan de Castuma E, Mattiazzi AR, Cingolani HE. Effect of hypercapnic acidosis on induction of arrhythmias by catecholamines in cat papillary muscles. Arch Int Physiol Biochem 85: 509–519, 1977. [DOI] [PubMed] [Google Scholar]
- 47.Terracciano CM, MacLeod KT. Effects of acidosis on Na+/Ca2+ exchange and consequences for relaxation in guinea pig cardiac myocytes. Am J Physiol Heart Circ Physiol 267: H477–H487, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol Heart Circ Physiol 276: H2168–H2178, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106: 1288–1293, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Xu L, Mann G, Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res 79: 1100–1109, 1996. [DOI] [PubMed] [Google Scholar]