Abstract
G protein-coupled receptor kinase 2 (GRK2) is a serine/theorinine kinase that phosphorylates and desensitizes agonist-bound G protein-coupled receptors. GRK2 is increased in expression and activity in lymphocytes and vascular smooth muscle (VSM) in human hypertension and animal models of the disease. Inhibition of GRK2 using the carboxyl-terminal portion of the protein (GRK2ct) has been an effective tool to restore compromised β-adrenergic receptor (AR) function in heart failure and improve outcome. A well-characterized dysfunction in hypertension is attenuation of βAR-mediated vasodilation. Therefore, we tested the role of inhibition of GRK2 using GRK2ct or VSM-selective GRK2 gene ablation in a renal artery stenosis model of elevated blood pressure (BP) [the two-kidney, one-clip (2K1C) model]. Use of the 2K1C model resulted in a 30% increase in conscious BP, a threefold increase in plasma norepinephrine levels, and a 50% increase in VSM GRK2 mRNA levels. BP remained increased despite VSM-specific GRK2 inhibition by either GRK2 knockout (GRK2KO) or peptide inhibition (GRK2ct). Although βAR-mediated dilation in vivo and in situ was enhanced, α1AR-mediated vasoconstriction was also increased. Further pharmacological experiments using α1AR antagonists revealed that GRK2 inhibition of expression (GRK2KO) or activity (GRK2ct) enhanced α1DAR vasoconstriction. This is the first study to suggest that VSM α1DARs are a GRK2 substrate in vivo.
Keywords: phenylephrine, hypertension, transgenic mice, gene ablation
hypertension is a substantial health problem in the Western world. In hypertensive patients, sympathetic nerves of the heart, kidneys, and skeletal muscle vasculature are activated, and sympathetic overactivity in the renal sympathetic outflow is a prominent pathophysiological feature (2, 6, 10). An increase in sympathetic nervous system activity results in an increase in catecholamines and, therefore, both α- and β-adrenergic receptor (AR) signaling. Vascular smooth muscle (VSM) ARs are essential to constriction and dilation of blood vessels and therefore play a major role in determining blood pressure (BP).
The predominant regulation of G protein-coupled receptors (GPCRs), such as βARs, occurs with the targeted phosphorylation of activated receptors leading to G protein uncoupling, a process termed desensitization. This process is initiated by phosphorylation of the agonist-occupied receptor by GPCR kinases (GRKs), a seven-member family (GRK1–7) of serine/threonine kinases (35, 36). GRKs possess in vivo substrate selectivity, and βARs are a target of GRK2 (29). VSM cells express all three α1AR subtypes: α1AAR, α1BAR, and α1DAR. We have previously shown that cardiac α1BAR is not desensitized by GRK2 phosphorylation in vivo (7), but the role of GRK2 phosphorylation for VSM α1BARs and the two other α1AR subtypes is not known.
Impairment in βAR-mediated dilation has been documented in patients with hypertension (11). Studies using animal models have also suggested a critical role of ARs and GRK2 in hypertension. There is a downregulation of the aorta βAR-adenylate cyclase system due to humoral and hemodynamic factors in vivo in spontaneously hypertensive rats (SHRs) and Dahl salt-sensitive rats (47). In addition, the coupling of βARs to ATP-sensitive K+ channels via the heterotrimeric Gs protein is compromised, thereby preventing K+ efflux and subsequent vasodilation (13). Increased GRK2 protein expression from lymphocytes and VSM of SHRs and Dahl salt-sensitive hypertensive rats as well as in lymphocytes of hypertensive patients has been correlated with increased BP (14–16). Finally, we created mice with VSM overexpression of GRK2 that had high BP, and their VSM βAR response was blunted (8).
Inhibition of GRK2 using a peptide inhibitor composed of the carboxyl-terminal portion of GRK2 (GRK2ct) has been an effective tool for restoring βAR responsiveness and rescuing heart failure in numerous animal models of the disease (17, 26, 37). Because βAR signaling is also compromised in high BP, in the present study, we assessed how inhibition of VSM GRK2, using either smooth muscle GRK2 gene ablation [GRK2 knockout (GRK2KO)] or VSM expression of a peptide inhibitor of GRK2 (GRK2ct ) (21, 28, 29), would affect the development of high BP using a renal artery stenosis model of hypertension. We found that inhibition of VSM GRK2 did not prevent the development of high BP. Although GRK2 inhibition either through peptide inhibition or gene ablation enhanced βAR-mediated dilation, it appears that vasoconstriction mediated through α1DARs is also augmented, suggesting that these receptors are in vivo substrates for GRK2.
MATERIALS AND METHODS
Construction and Characterization of Transgenic Mice
We used two different transgenic mouse models in this study: VSM-specific GRK2KO and VSM GRK2ct mice.
VSM GRK2KO.
Floxed GRK2 mice were constructed with loxP sites flanking exons 3–6 of GRK2 (31). By mating transgenic smooth muscle-specific Cre recombinase expressing (sm-Cre-IRES-eGFP mice kindly provided by Dr. Kotlikoff) (56) and GRK2 floxed mice (31), we generated VSM GRK2KO mice. Control mice were nontransgenic littermates from the Cre-floxed GRK2 hybrid line.
VSM GRK2ct.
As described previously (8, 22, 24), a 481-bp portion of the SM22α promoter was fused to the carboxyl terminal of the coding sequence of bovine GRK2, a 582-bp portion of GRK2 shown to competitively inhibit the actions of GRK2 by binding to Gβγ and preventing the translocation of GRK2 to the membrane, with an inability to elicit desensitization (28, 29), and a modified Simian virus 40 intron poly(A+) signal (8, 22, 24, 29). The VSM-GRK2ct transgene underwent pronuclear injection in C57BL/6 embryos at the Duke Comprehensive Cancer Center Transgenic Facility. Two independent transgenic founder lines were verified and maintained as heterozygotes on the C57BL/6 background. Control mice were nontransgenic littermates.
Determination of RNA Expression
Eight aortas were pooled, and VSM layers were digested enzymatically and separated from the adventitial layer after mechanical denudation of the endothelial cell layer (9). Frozen samples were homogenized, and total RNA extraction from the VSM was performed according to the manufacturer's protocol (Qiagen RNeasy and DNase Kit, Qiagen). The Bio-Rad iScript kit (Bio-Rad, Hercules, CA) was used to synthesize cDNA from 0.5 μg of total RNA. SYBR green (Bio-Rad) was used for quantitative RT-PCR. The primers used was as follows: GRK2, forward 5′-ATGCATGGCTACATGTCCAA-3′ and reverse 5′-ATCTCCTCCATGGTCAGCAG-3′; GRK5, forward 5′-ACCTGAGGGGAGAACCATTC-3′ and reverse 5′-TGGACTCCCCTTTCCTCTTT-3′; GRK3, forward 5′-TGGCTACATGTCCAAGA-3′ and reverse 5′-GTTCCGTCGTTTTACGG-3′; and, as a control gene, 28S, forward 5′-TTGAAAATCCGGGGGAGAG-3′ and 5′-ACATTGTTCCAACATGCCAG-3′ (33). mRNA expression was determined. Melt-curve analysis, verification of linear range amplification, and sequencing of PCR products were completed for all RT-PCR. Data were analyzed by the 2−ΔΔCt method, where Ct is the threshold cycle, ΔΔCt = ΔCt(sample) − ΔCt(normalization gene), and ΔCt is the Ct of the target gene subtracted from the Ct of the housekeeping gene (30, 46). Gene expression of α1AR subtypes in mouse thoracic aortic vessels was performed with and without enzymatic digestion as described above using oligonucleotide primers as previously described (45).
Conscious BP Measurements
Mice were anesthetized with ketamine (50 mg/kg) and xylazine (2.5 mg/kg), in-dwelling catheters (PA-C10, Data Sciences) were inserted into the left common carotid artery, and battery devices were stored in the subcutaneous subscapular layer. Mice were allowed to recover, and BP on conscious, unrestrained mice was measured 4 days postsurgery. Systolic BP (SBP), diastolic BP (DBP), and heart rate were measured. Mean arterial pressure (MAP) was calculated as DBP + 1/3(SBP − DBP). Two-minute BP recordings from mice in each experimental group were measured hourly over a 24-h period; recordings were then integrated and averaged.
Histology and Morphology
Paraffin-fixed thoracic aortas were sectioned and processed for immunohistochemisty using a commercially available primary antibody to GRK2 (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (8). Aortic wall thickness was determined by measuring the perpendicular distance between the internal and external elastic lamina at 10 different locations around the entire circumference of each hematoxylin and eosin-stained blood vessel. The 10 different thicknesses were then averaged for each vessel and counted as a sample size of 1 for GRK2KO, GRK2ct, and control groups. Heart-to-body weight ratios (in mg/g) were calculated from weighing mice before death and then weighting hearts after dissection.
Two-Kidney, One-Clip Surgeries
Mice were anesthetized as described above, the left kidney, renal artery, and vein were exposed via a lower left flank incision, and careful separation of the renal artery and vein enabled the placement of a stainless steel U-shaped clip (0.12 mm inner diameter) to be placed around the renal artery (18, 54). 5-0 vicryl sutures closed the incision. Sham operations were identical except that the clip was removed prior to incision closure. Two kidney, one clip (2K1C) renovascular hypertension success was verified by an elevated left-to-right kidney ratio as determined by weighing each kidney upon death. Mice were treated with ibuprofen (10 mg/50 ml) via drinking water for 48 h after surgery. All protocols were approved and performed in accordance with Thomas Jefferson University Institutional Animal Care and Use Committee regulations. All mouse groups were studied 4 wk postsurgery. Blood was drawn from the abdominal aorta of anesthetized sham and 2K1C mice and centrifuged for plasma isolation. ELISA was performed to determine catecholamine levels in these mice (R&D Systems).
BP with Acute Agonist Stimulation
Mice were anesthetized with ketamine and xylazine, and an in-dwelling radiotelemetry catheter was inserted into the left common carotid as described above. Another catheter (PE-10) was inserted into the right jugular vein. The venous access catheter was flushed with 50 μl of heparinized PBS (30 U/ml). Agonist infusions were performed in mice when heart rates rose above 400 beats/min. Mice received a bolus injection of drug at 2- to 5-min intervals, and a maximum of four drug-response curves were performed per animal. The recordings from each animal are depicted as peak responses and were normalized to baseline reading as 100% to account for the variability in sedation.
Vascular Reactivity Experiments
Thoracic aortas were dissected, and 2.5-mm segments were hung on a force pressure transducer as described previously (8, 24). Rings were treated with 100 μM N-nitro-l-arginine methyl ester (l-NAME) 10 min prior to agonist treatment to remove endothelium-derived nitric oxide-mediated effects. The absence of endothelium-dependent relaxation to a dose of ACh (10−5 M) confirmed inhibition. Thoracic aorta rings were then exposed to cumulative increasing concentrations of isoproterenol (Iso) or phenylephrine (PE) in the presence or absence of various inhibitors as described. For the Iso concentration-response curve, a dose of 3 × 10−7 M PE was used to generate preconstriction. For ANG II responses, abdominal aortic rings were dissected and used since the ANG II response is limited in the thoracic aorta. Data were obtained and analyzed (Chart 5.0), and tension was plotted normalized to maximum constriction derived at 100 μM PE. Inhibitors include prazosin (Sigma), AH11110A (Sigma), chloroethylclonidine (CEC; Sigma), WB4101 (Tocris), and BMY-7378 (Sigma).
Statistical Analysis
Data are expressed as means ± SE. Data were analyzed using one-way ANOVA, two-way ANOVA, or two-tailed unpaired Student's t-tests as indicated. Curve-fit nonlinear regression analysis was used to obtain sigmoidal dose-response curves as well as EC50 and negative logarithm of the dissociation constant (pA2) values (GraphPadPrism, GraphPad Software, San Diego, CA).
RESULTS
Mice
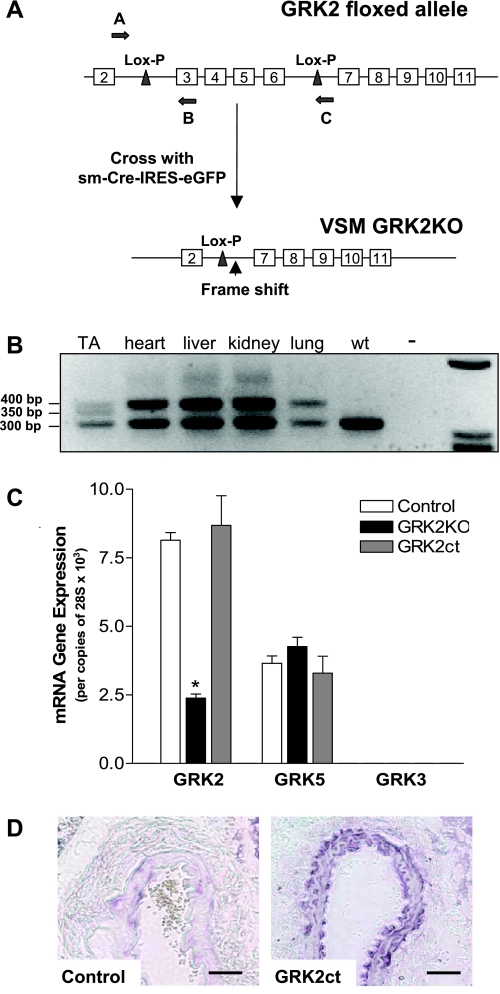
VSM-specific GRK2KO and GRK2ct peptide inhibitor (GRK2ct) age-matched mice were used in these experiments. Control mice were as described in materials and methods. PCR genotyping of heterozygous GRK2KO mice with the oligonucleotide primers shown in Fig. 1A confirmed GRK2 deletion of exons 3–6 was specific to smooth muscle (Fig. 1B). Quantitative RT-PCR demonstrated a 75% reduction in GRK2 levels in the VSM layer of the thoracic aorta isolated from our GRK2KO mouse (Fig. 1C). GRK5 and GRK3 (the second and third most abundant GRKs in the VSM) levels remained unchanged. The amount of abundance in VSM was GRK2 > GRK5 >> GRK3.
Fig. 1.
Vascular smooth muscle (VSM)-specific G protein-coupled receptor kinase 2 (GRK2) knockout (GRK2KO) and GRK2ct peptide inhibitor transgenic mouse lines. A: cartoon of Lox-P sites within the GRK2 floxed transgene and the frame shift that occurs when mating GRK2 floxed mice with smooth muscle Cre recombinase-expressing mice (sm-Cre-IRES-eGFP). Primers used for screening are indicated by arrows A–C. B: PCR genotyping of a heterozygous mouse confirming GRK2 deletion of exons 3–6 is specific to smooth muscle in the thoracic aorta (TA) and maintains a floxed GRK2 allele in the heart, liver, kidney, and lung compared with wild-type (wt) mice. C: quantitative real-time PCR with primers to the amino-terminal portion of GRK2, GRK5, and GRK3. GRK3 expression is too low to be visible on the y-axis used. VSM from the aorta of 8 mice were pooled and considered as n = 1. n = 6 for each group. *P < 0.05 vs. control by one-way ANOVA and Bonferroni post t-test. D: immunohistochemisty from a cross-sectional slice through the common carotid artery of control and VSM GRK2ct mice. The GRK2 antibody recognizes the carboxyl-terminal portion of GRK2 and therefore detects both GRK2ct and endogenous levels of GRK2. Bar = 50 μm.
VSM-specific expression in two independent GRK2ct transgenic founder lines was verified by quantitative RT-PCR for RNA, protein immunoblot analysis, and immunohistochemistry (Fig. 1D).
BP, VSM Thickness, and Cardiac Size Are Unchanged in Mice Lacking Functional VSM GRK2
At rest, under basal conditions, GRK2KO and GRK2ct mice were normotensive (Table 1). To determine whether our VSM-specific alterations changed the normal morphology of blood vessels, we isolated the thoracic aorta from control, GRK2KO, and GRK2ct mice. VSM thickness was measured, and no significant change was noted (Table 1). A common consequence of altered vascular control is cardiac hypertrophy, and there were no discernable differences in heart size among the three groups (Table 1).
Table 1.
General cardiovascular characteristics of two mouse models of VSM-specific GRK2 inhibition: GRK2KO and GRK2ct
| Output | Control | GRK2KO | GRK2ct |
|---|---|---|---|
| MAP, mmHg | 107.3±2.5 (9) | 107.4±1.9 (8) | 103.1±1.5 (10) |
| VSM thoracic aorta thickness, μm | 28.3±0.7 (6) | 27.6±0.8 (10) | 293.5±1.8 (7) |
| Heart-to-body weight ratio, mg/g | 4.90±0.70 (6) | 4.86±0.23 (10) | 4.80±0.16 (7) |
Data are presented as means ± SE; numbers in parentheses are numbers of mice. VSM, vascular smooth muscle; GRK2KO, G protein-coupled receptor kinase 2 (GRK2) knockout; GRK2ct, peptide inhibitor encoding the carboxyl-terminal portion of GRK2; MAP, mean arterial pressure.
Although GRK2 Is Increased After Renal Artery Stenosis, Inhibition of GRK2 Does Not Prevent 2K1C Hypertension
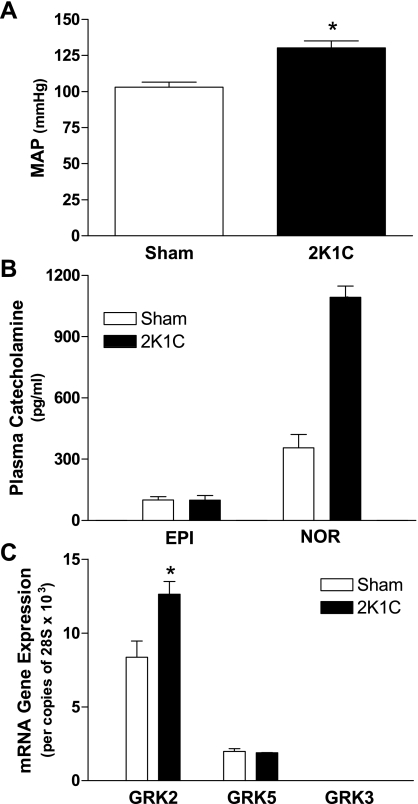
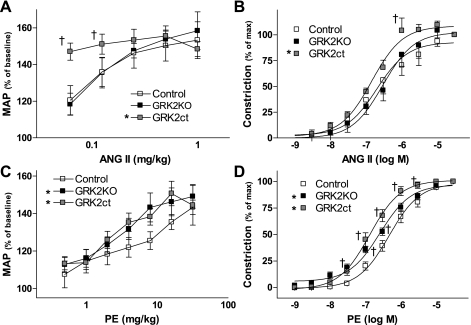
Conscious MAP was increased ∼30% in control mice 4 wk following renal artery stenosis surgery compared with sham controls (Fig. 2A). Catecholamine levels were measured in plasma isolated from sham and 2K1C mice. There were no differences in epinephrine levels between sham and 2K1C mice (Fig. 2B). In contrast, norepinephrine levels were elevated roughly threefold upon renovascular hypertension (Fig. 2B). We determined GRK2, GRK5, and GRK3 mRNA expression in the VSM layer of the thoracic aorta isolated from sham and 2K1C mice. GRK2 mRNA expression was elevated ∼50% in isolated VSM from 2K1C mice compared with sham animals, whereas GRK5 and GRK3 levels remained unchanged (Fig. 2C).
Fig. 2.
Renal artery stenosis using the two-kidney, one-clip (2K1C) model increases mean arterial pressure (MAP), plasma norepinephrine (NOR), and VSM GRK2 expression. A: MAP in sham and 2K1C control mice. n = 8 for each. *P < 0.05 by an unpaired two-tailed Student's t-test. B: epinephrine (EPI) and norepinephrine levels were measured in plasma isolated from sham and 2K1C control mice. n = 5 for each. C: quantitative RT-PCR determined GRK2, GRK5, and GRK3 mRNA expression in sham and 2K1C control mice. GRK3 mRNA abundance was very low and not readily detectable on the y-axis scale used. n = 4 for each. *P < 0.05 vs. control by one-way ANOVA and Bonferroni's post t-test.
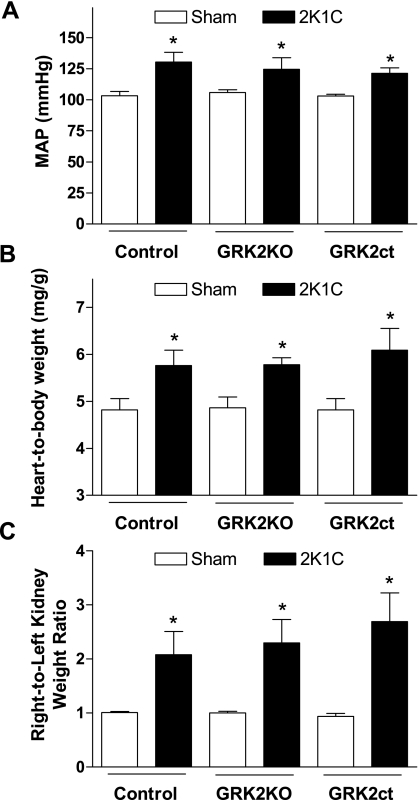
Based on the result that GRK2 expression was increased in our animal model of hypertension (Fig. 2C), our previous results that increased VSM GRK2 expression was sufficient to increase BP (8), and the hypothesis that alterations in GRK2 levels could have a profound impact on the constriction status of blood vessels during high BP, we performed the 2K1C surgical model in our GRK2KO and GRK2ct transgenic mouse lines. When hypertension was surgically induced, BP was increased similarly in both GRK2KO and GRK2ct mice compared with their respective control mice (Fig. 3A). Heart-to-body weight ratios were similar (Fig. 3B) and left-to-right kidney ratios indicated comparable renal artery stenosis (Fig. 3C). These data suggest that the mouse groups underwent a similar amount of renovascular stricture.
Fig. 3.
VSM GRK2 inhibition fails to rescue the 2K1C model of high blood pressure (BP). A: 4-wk postsurgical mice were assessed for conscious MAP for control (sham: n = 12 and 2K1C: n = 5), GRK2KO (sham: n = 10 and 2K1C: n = 6), and GRK2ct (sham: n = 10 and 2K1C: n = 6) mice. B: cardiac hypertrophy was measured as heart-to-body weight ratios for all groups. C: all three 2K1C mouse groups had increased right-to-left kidney weight ratios. *P < 0.05 vs. respective sham mice by one-way ANOVA and Bonferroni's post t-test.
Inhibition of VSM GRK2 Enhances βAR Signaling
No significant differences in vascular responses were noted for any of the ligands tested (data not shown) between nontransgenic littermate siblings from either GRK2KO or GRK2ct groups; therefore, control mice are shown as one group for clarity.
It has been extensively studied in the heart that GRK2 inhibition enhances βAR signaling (26, 29, 31). We found that BP increases seen upon renal artery ligation were not reduced with either peptide inhibition or gene ablation of GRK2. Therefore, we investigated βAR signaling in control mice and mice with GRK2 inhibition.
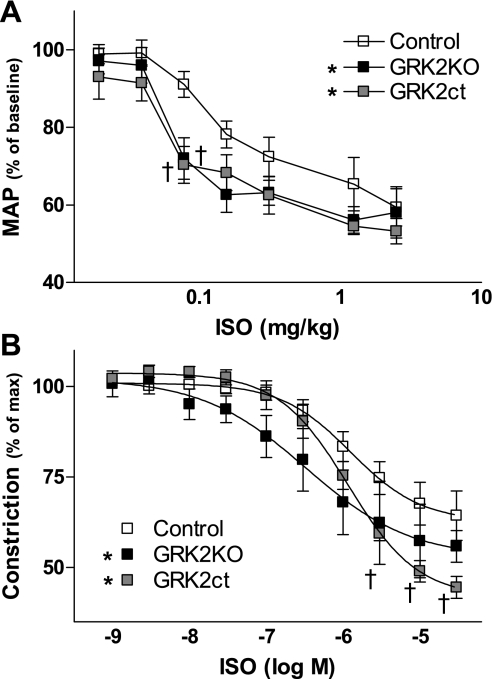
Acute agonist infusion through the jugular vein with simultaneous anesthetized BP measurement demonstrated an increased responsiveness to the βAR agonist Iso in GRK2KO and GRK2ct mice compared with controls (Fig. 4A). We performed Iso concentration-response curves in the isolated thoracic aorta in the presence of l-NAME to inhibit nitric oxide synthase activity and prevent endothelial cell-related nitric oxide release. We found in control mice that the pD2 (−log EC50) value for Iso (5.91 ± 0.17, n = 6; Fig. 4B) obtained in the present study corresponded well with what has been reported (24, 43). There was a significant left shift in the response to Iso in GRK2KO mice (pD2 = 6.51 ± 0.26, n = 6; Fig. 4B). In GRK2ct mice, although the EC50 was similar to control (pD2 = 5.94 ± 0.08, n = 5), there was a significant 50% increase in maximum dilation in response to Iso (Fig. 4B). Therefore, although VSM GRK2 inhibition, either through expression or activity, was not sufficient to rescue a model of hypertension, we did observe an increase in βAR-mediated vasodilation in these mice.
Fig. 4.
Enhanced in vivo and in situ β-adrenergic receptor (AR) signaling in both GRK2KO and GRK2ct mice compared with controls. A: acute agonist infusion of isoproterenol (Iso) through the jugular vein with simultaneous anesthetized BP tracing was performed when heart rates rose above 400 beats/min in control (n = 5), GRK2KO (n = 5), and GRK2ct (n = 5) mice. MAP was normalized to the baseline reading, which was considered as 100%. B: in situ vascular reactivity experiments on TA segments in control (n = 5), GRK2KO (n = 5), and GRK2ct (n = 6) vessels. Tension was normalized (100%) to the maximal response of a concentration of 3 × 10−7 M phenylephrine (PE). Nitric oxide synthase activity was inhibited using N-nitro-l-arginine methyl ester (l-NAME) to prevent endothelial cell release of nitric oxide and verified with a lack of response to 10−5 M ACh. *P < 0.05 vs. GRK2KO by two-way ANOVA with respect to dose and control; †P < 0.05 vs. control by Bonferroni's post t-test.
ANG II and α1AR Signaling Are Enhanced in GRK2KO and GRK2ct VSM
To uncover why enhancement of βAR-mediated dilation did not reduce BP upon 2K1C-induced hypertension, we examined acute BP responses to stimulation of two different receptors that elicit vasoconstriction: ANG II receptors and α1ARs. There was a concentration-dependent increase in BP in response to ANG II in control mice (Fig. 5A). The response was similar in GRK2KO mice (Fig. 5A). In contrast, at the two lowest concentrations of ANG II tested, there was a significantly enhanced increase in BP in GRK2ct mice (Fig. 5A). We also documented a similar relationship in vascular reactivity experiments of the abdominal aorta such that the ANG II response in GRK2KO vessels was unchanged but enhanced in GRK2ct vessels (Fig. 5B).
Fig. 5.
Enhanced in vivo and in situ α1AR and ANG II receptor signaling. A: acute agonist infusion revealed that ANG II-induced increases in BP were enhanced in GRK2ct mice, but not GRK2KO mice, compared with control animals. n = 5 each. B: vasoconstriction in vascular reactivity experiments in abdominal aorta segments from control (n = 5), GRK2KO (n = 5), and GRK2ct (n = 6) vessels. C: PE-induced elevations in BP in control (n = 5), GRK2KO (n = 5), and GRKct (n = 6) mice. D: PE-induced vasoconstriction in control (n = 11), GRK2KO (n = 17), and GRK2ct (n = 12) TA segments. Tension normalized to the 10−5 M response. *P < 0.05 vs. GRK2KO by two-way ANOVA with respect to dose and control; †P < 0.05 vs. control by Bonferroni's post t-test.
We also examined the response to PE, another potent vasoconstrictor. The acute BP response to PE was enhanced in both GRK2KO and GRK2ct mice (Fig. 5C). We also saw a significant left shift in constriction in the concentration response to PE in both GRK2KO (pD2 = 6.59 ± 0.05, n = 17, P = 0.0023 vs. control by a two-tailed, unpaired Student's t-test) and GRK2ct (pD2 = 6.88 ± 0.05, n = 12, P < 0.0001 vs. control by two-tailed, unpaired Student's t-test) thoracic aortas compared with control (pD2 = 6.34 ± 0.05, n = 11) thoracic aortas (Fig. 5D). Therefore, it appears that VSM α1ARs are affected by GRK2 inhibition.
α1AR Subtype Distribution in Mouse VSM
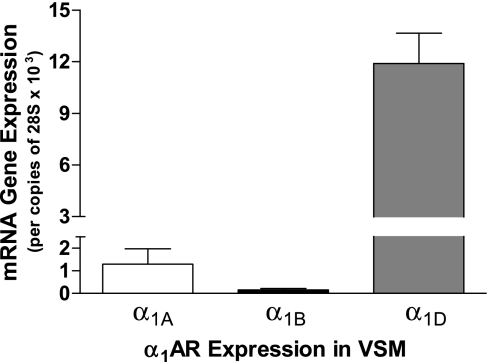
We verified the α1AR subunits present in mouse VSM using quantitative RT-PCR on control mouse thoracic aortas. Intact aorta blood vessels, which contain a primarily VSM layer, endothelial cells, and adventitia (primarily comprised of fibroblasts), were enzymatically digested following mechanical scraping to remove endothelial cells. The adventitial layer was stripped, and RNA was isolated from the VSM layer. In VSM, α1DAR was the most abundant α1AR subtype by >10-fold over the α1AAR subtype and 100-fold over the α1BAR subtype (Fig. 6).
Fig. 6.
α1AR subtype gene expression in VSM isolated from mouse TAs. Blood vessels were analyzed for α1AR subtype expression via quantitative RT-PCR. Enzymatic removal of adventitia and mechanical scraping of endothelial cells isolated the VSM-only tunica media layer (8 aorta pooled and repeated 4 times).
α1BAR Is Not Involved in Mouse Thoracic Aorta Constriction
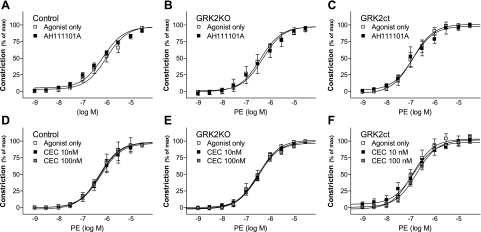
To determine the role of GRK2 inhibition and α1BAR in vasoconstriction, we examined the effects of two α1BAR inhibitors: AH11110A and CEC (Fig. 7). Neither α1BAR antagonist had any effect on PE-induced constriction in control, GRK2KO, or GRK2ct mice (Fig. 7 and Table 2), and augmented constriction to PE in GRK2KO and GRK2ct blood vessels was maintained. These data suggest α1BARs are not involved in vasoconstriction of the mouse thoracic aorta, similar to what others have seen in other large mouse blood vessels (19).
Fig. 7.
α1BAR inhibition does not alter enhanced in situ α1AR signaling in GRK2-inhibited vessels. Responses to PE and α1BAR antagonists in TAs were taken from control (A and D), GRK2KO (B and E), and GRK2ct (C and F) mice. All inhibitors were added 10 min prior to stimulation. A–C: control, GRK2KO, and GRK2ct vessels in the presence of the α1BAR inhibitor AH11110A (1 μM). D–F: additional experiments with another α1BAR inhibitor, chloroethylclonidine (CEC). n = 4–7 for all groups.
Table 2.
Antagonist profile in the mouse thoracic aorta
| Antagonist Agent | AR Subtype |
pA2 |
||
|---|---|---|---|---|
| Control | GRK2KO | GRK2ct | ||
| AH11110A | α1BAR | ND | ND | ND |
| CEC | α1BAR | ND | ND | ND |
| WB4101 | α1AAR | 8.38±0.25 (5) | 8.94±0.23 (5) | 8.89±0.20 (6) |
| BMY-7378 | α1DAR | 7.06±0.19 (5) | 7.00±0.25 (5) | 7.61±0.22 (4) |
Data are presented as means ± SE; numbers in parentheses are numbers of mice. AR, adrenergic receptor; pA2, measure of antagonist potency; CEC, chloroethylclonidine. Where antagonists did not cause a shift in the phenylephrine concentration response, pA2 could not be determined (ND).
α1DARs Mediate Mouse Thoracic Aorta Constriction and Are Likely Targets of GRK2
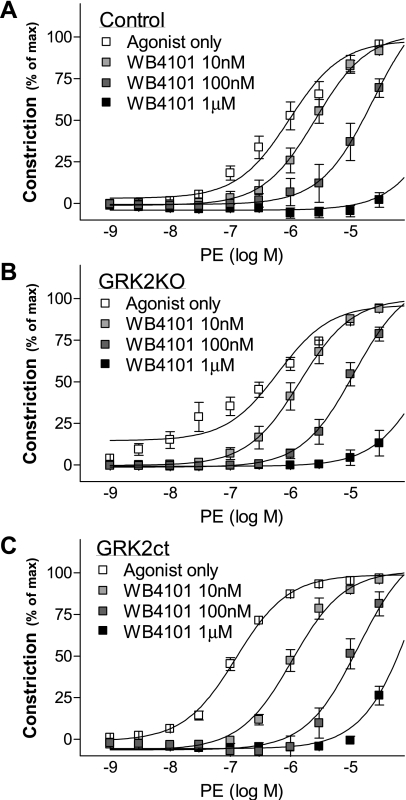
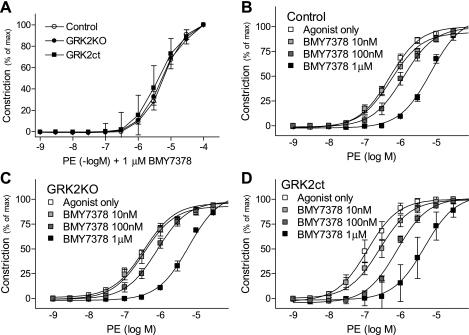
To test the involvement of α1AAR, we used WB4101, a potent α1AR antagonist with slight selectivity for α1AAR. WB4101 caused a right shift in the concentration response to PE in control, GRK2KO, and GRK2ct vessels (Fig. 8). However, the pA2 values (Table 2) suggest that it is likely effects due to α1DAR antagonism rather than α1AAR (34), although we cannot definitively rule out the involvement of α1AARs. In the presence of BMY-7378, an α1DAR antagonist, there was a right shift in the concentration response to PE (Fig. 9). In addition, no longer was the response of the GRK2KO or GRK2ct aorta significantly greater than the control (Table 2 and Fig. 9A). These data, coupled with our quantitative RT-PCR data, suggest that α1DARs mediate vasoconstriction in mouse thoracic aorta VSM and that the lack of GRK2 activity enhances constriction through α1DARs.
Fig. 8.
Vasoconstriction to PE in the presence of WB4101, an α1AAR inhibitor. A–C: responses to PE in the TA taken from control (A), GRK2KO (B), and GRK2ct (C) mice. n = 4–7 for all groups.
Fig. 9.
BMY-7378, an α1DAR inhibitor, restored normal α1AR vasoconstriction in GRK2KO and GRK2ct TAs. A: PE constriction in the mouse TA isolated from control, GRK2KO, and GRK2ct mice in the presence of the α1DAR antagonist BMY-7378 (1 μM). B–D: PE constriction in the mouse TA taken from control (B), GRK2KO (C), and GRK2ct (D) mice. n = 4–13 for all groups.
DISCUSSION
We have previously reported that enhanced VSM GRK2 expression is related to high BP and diminished βAR-mediated dilation (8). In the present study, we documented that renal artery stenosis, a model of hypertension, is associated with increased plasma norepinephrine levels and increased VSM GRK2 expression. We were interested in the possibility that inhibition of GRK2, either through VSM-specific gene ablation or using VSM expression of a peptide inhibitor of GRK2, GRK2ct, could enhance βAR dilation sufficiently to prevent high BP in the 2K1C model. VSM GRK2 inhibition, either through expression or activity, was not sufficient to prevent high BP in the 2K1C model. This finding was somewhat surprising as we verified that in vivo βAR-mediated dilation was improved. We have previously shown that GRK2 did not desensitize cardiac α1BARs (7). The observation that there was an increase in vasoconstriction in response to VSM α1AR stimulation was unexpected. Our data herein suggest that α1DARs are likely targets of GRK2-mediated desensitization, and we confirmed our previous findings (7) that VSM α1BARs are also not substrates of GRK2 in vivo.
α1DARs have been implicated in the pathogenesis and/or maintenance of hypertension (20, 48, 51, 52). However, both α1DAR (49) and α1AAR (42) knockout mice, but not α1BAR knockout mice (4), are hypotensive, suggesting a prominent role of both α1DARs and α1AARs in blood vessel regulation and, therefore, BP control. Localization experiments of the α1AAR (42), our data herein, and the data of others suggest that the most abundant α1AR subtype in the VSM layer of the mouse thoracic aorta is the α1DAR (19, 38, 45, 57), suggesting that α1DARs confer the majority of vasoconstriction, at least in the mouse aorta (5, 49). Given these observations, it is likely that the effects we observed are due to an interaction of α1DAR and GRK2. However, we cannot definitively rule out regulation of the α1AAR by GRK2 since WB4101 has weak selectivity and may be acting at both α1DARs as well as α1AARs (57), and this warrants further investigation. However, our data are clear that α1BAR mediated vasoconstriction was unchanged by VSM GRK2 inhibition since two different α1BAR antagonists, AH11110A and CEC, had no affect on vasoconstriction in the mouse thoracic aorta.
It has been suggested that α1DARs can form heterodimers with β2ARs (32, 50). Previous data have suggested that βAR-α1DAR dimerization does not have any affect on vasoconstriction mediated through the α1DAR subtype (12). In addition, data have also suggested that the β1AR is the primary vasodilator in the mouse (1). It is unlikely that GRK2 is mediating its effect on α1DARs through the β2AR in our system, although this remains to be directly addressed.
For the majority of data represented in this study, we observed that the effects of gene ablation of GRK2 were similar to peptide inhibition of GRK2 using GRK2ct. This suggests that although GRK2ct acts as a Gβγ sequestrant, the primary mode of action, at least for the outputs we measured, was through GRK2 inhibition. However, we did observe differences in the response to ANG II stimulation. Gene ablation of GRK2 had no effect, whereas GRK2ct enhanced, ANG II-mediated constriction. Although α1ARs are thought to mostly couple to Gq, ANG II is known to couple to both Gq and Gi. It is known that GRK2ct affects Gi-mediated signaling (27), and it warrants further investigation to determine if the differential effects on ANG II-mediated constriction are due to its ability to couple to Gi. It is also known that diminishing GRK2 expression, at least in vitro, enhances arrestin-mediated ERK activation induced by GRK5 phosphorylation of ANG II type 1 receptors (25). The role that this alternative “nonclassical” signaling plays in vivo in VSM has yet to be determined. GRK2ct could also be inhibiting GRK3 activity, since similar Gβ pools have been implicated in GRK2- and GRK3-mediated translocation to the plasma membrane (3). It is also possible, but unlikely, that differences could be due to the Cre recombinase expression being driven by the smooth muscle myosin heavy chain promoter, therefore expressed in all smooth muscle (56), whereas GRK2ct is only expressed in VSM, as determined by a portion of the SM22α promoter (8, 22, 24). Further investigation into the similarities and differences between inhibition of GRK2 through either expression or activity may be warranted to determine the full extent of the actions of GRK2ct.
The usefulness of GRK2ct as a tool to improve heart failure outcome has been extensively studied (17, 23, 26, 40). In cardiac myocytes, the improvement of βAR signaling is sufficient to repair cardiac contractility. For this reason, it was surprising that restoration of VSM βAR dilation was not sufficient to reduce high BP in a hypertensive model. In cardiac myocytes, α1BAR signaling predominates, and it is thought to be more likely associated with hypertrophy rather than function (55) and a target of GRK3 (7, 53). In addition, in contrast to VSM, where α1DARs directly contribute to vasoconstriction, α1DARs on cardiac myocytes do not play a significant role in contraction (41, 44). Therefore, the relative role and importance that α1DARs play in VSM versus cardiac myocytes are likely important reasons why the α1DAR effect of GRK2ct has not been documented to affect cardiac function.
Another reason why VSM GRK2 inhibition may not have been sufficient to rescue hypertension could be because vascular endothelial cells also express βARs and their stimulation leads to nitric oxide release and subsequent vasodilation (39). Similar to VSM, the endothelial βAR response is likely also desensitized in high BP. Therefore, it is possible that in vivo the potentially diminished endothelial βAR response has a profound effect such that the restoration of VSM βAR signaling is not sufficient to overcome the diminished βAR signaling of endothelial cells. Importantly, however, stimulation of α1ARs on endothelial cells does not contribute to vasoconstriction. Therefore, it is possible that endothelium-specific GRK2 inhibition might be a successful strategy to reduce high BP, although this remains to be determined.
In summary, we describe that the renal artery stenosis model of high BP in mice is accompanied by increased plasma norepinephrine levels and an increase in VSM GRK2, but not GRK3 or GRK5, expression. Surprisingly, although inhibition of VSM-specific GRK2 (either through gene ablation or peptide inhibition) enhanced βAR-mediated dilation, it was not sufficient to rescue a renal stenosis model of hypertension. We identify that following VSM GRK2 inhibition, α1DAR stimulation is increased, which is likely the mechanism preventing the restoration of normal BP: the increase in βAR-mediated dilation is balanced by an increase in α1AR constriction. Our data suggest that GRK2 inhibition has effects on multiple VSM GPCRs that contribute to both vasoconstriction and vasodilation.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant RO1-HL-069847 (to A. D. Eckhart), by the W. W. Smith Charitable Trust (to A. D. Eckhart), and by an American Heart Association Predoctoral Fellowship (to H. I. Cohn). This project was funded in part under a grant with the Pennsylvania Department of Health. The Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking β1- or β2-adrenergic receptors. Mol Pharmacol 60: 955–962, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Corry DB, Tuck ML. Obesity, hypertension, and sympathetic nervous system activity. Curr Hypertens Rep 1: 119–126, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. Receptor and Gβγ isoform-specific interactions with G protein-coupled receptor kinases. Proc Natl Acad Sci USA 94: 2180–2185, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, McGrath JC. A knockout approach indicates a minor vasoconstrictor role for vascular α1B-adrenoceptors in mouse. Physiol Genomics 9: 85–91, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Deng XF, Chemtob S, Varma DR. Characterization of α1D-adrenoceptor subtype in rat myocardium, aorta and other tissues. Br J Pharmacol 119: 269–276, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeQuattro V, Li D. Sympatholytic therapy in primary hypertension: a user friendly role for the future. J Hum Hypertens 16, Suppl 1: S118–S123, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res 86: 43–50, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates β-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol 61: 749–758, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Eckhart AD, Zhu Z, Arendshorst WJ, Faber JE. Oxygen modulates α1B-adrenergic receptor gene expression by arterial but not venous vascular smooth muscle. Am J Physiol Heart Circ Physiol 271: H1599–H1608, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Esler M Differentiation in the effects of the angiotensin II receptor blocker class on autonomic function. J Hypertens Suppl 20: S13–S19, 2002. [PubMed] [Google Scholar]
- 11.Feldman RD, Gros R. Impaired vasodilator function in hypertension: the role of alterations in receptor-G protein coupling. Trends Cardiovasc Med 8: 297–305, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Cazarin ML, Smith JL, Olszewski KA, McCune DF, Simmerman LA, Hadley RW, Kraner SD, Piascik MT. The α1D-adrenergic receptor is expressed intracellularly and coupled to increases in intracellular calcium and reactive oxygen species in human aortic smooth muscle cells. J Mol Signal 3: 6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto K, Fujii K, Abe I. Impaired β-adrenergic hyperpolarization in arteries from prehypertensive spontaneously hypertensive rats. Hypertension 37: 609–613, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gros R, Benovic JL, Tan CM, Feldman RD. G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest 99: 2087–2093, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gros R, Chorazyczewski J, Meek MD, Benovic JL, Ferguson SS, Feldman RD. G-Protein-coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension 35: 38–42, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Gros R, Tan CM, Chorazyczewski J, Kelvin DJ, Benovic JL, Feldman RD. G-protein-coupled receptor kinase expression in hypertension. Clin Pharmacol Ther 65: 545–551, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JL, Theilade J, Aplin M, Sheikh SP. Role of G-protein-coupled receptor kinase 2 in the heart–do regulatory mechanisms open novel therapeutic perspectives? Trends Cardiovasc Med 16: 169–177, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD. Vascular smooth muscle Gq signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol Heart Circ Physiol 293: H3072–H3079, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hosoda C, Tanoue A, Shibano M, Tanaka Y, Hiroyama M, Koshimizu TA, Cotecchia S, Kitamura T, Tsujimoto G, Koike K. Correlation between vasoconstrictor roles and mRNA expression of α1-adrenoceptor subtypes in blood vessels of genetically engineered mice. Br J Pharmacol 146: 456–466, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarra M, Pardo JP, Lopez-Guerrero JJ, Villalobos-Molina R. Differential response to chloroethylclonidine in blood vessels of normotensive and spontaneously hypertensive rats: role of α1D- and α1A-adrenoceptors in contraction. Br J Pharmacol 129: 653–660, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inglese J, Koch WJ, Touhara K, Lefkowitz RJ. Gβγ interactions with PH domains and Ras-MAPK signaling pathways. Trends Biochem Sci 20: 151–156, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Keys JR, Greene EA, Koch WJ, Eckhart AD. Gq-coupled receptor agonists mediate cardiac hypertrophy via the vasculature. Hypertension 40: 660–666, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Keys JR, Koch WJ. The adrenergic pathway and heart failure. Recent Prog Horm Res 59: 13–30, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation 112: 1145–1153, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA 102: 1442–1447, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch WJ Genetic and phenotypic targeting of β-adrenergic signaling in heart failure. Mol Cell Biochem 263: 5–9, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by Gβγ activation of p21ras. Proc Natl Acad Sci USA 91: 12706–12710, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the βγ subunits of heterotrimeric G proteins on the β-adrenergic receptor kinase. J Biol Chem 268: 8256–8260, 1993. [PubMed] [Google Scholar]
- 29.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science 268: 1350–1353, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW 2nd. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and β-adrenergic signaling. Circ Res 99: 996–1003, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Milligan G G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta 1768: 825–835, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Mullick A, Elias M, Harakidas P, Marcil A, Whiteway M, Ge B, Hudson TJ, Caron AW, Bourget L, Picard S, Jovcevski O, Massie B, Thomas DY. Gene expression in HL60 granulocytoids and human polymorphonuclear leukocytes exposed to Candida albicans. Infect Immun 72: 414–429, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pallavicini M, Budriesi R, Fumagalli L, Ioan P, Chiarini A, Bolchi C, Ugenti MP, Colleoni S, Gobbi M, Valoti E. WB4101-related compounds: new, subtype-selective α1-adrenoreceptor antagonists (or inverse agonists?). J Med Chem 49: 7140–7149, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F Jr. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res 69: 46–56, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med 10: 81–89, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Petrofski JA, Koch WJ. The β-adrenergic receptor kinase in heart failure. J Mol Cell Cardiol 35: 1167–1174, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Piascik MT, Guarino RD, Smith MS, Soltis EE, Saussy DL Jr, Perez DM. The specific contribution of the novel α-1D adrenoceptor to the contraction of vascular smooth muscle. J Pharmacol Exp Ther 275: 1583–1589, 1995. [PubMed] [Google Scholar]
- 39.Queen LR, Ferro A. β-Adrenergic receptors and nitric oxide generation in the cardiovascular system. Cell Mol Life Sci 63: 1070–1083, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature 415: 206–212, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Rohde S, Sabri A, Kamasamudran R, Steinberg SF. The α1-adrenoceptor subtype- and protein kinase C isoform-dependence of norepinephrine's actions in cardiomyocytes. J Mol Cell Cardiol 32: 1193–1209, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Rokosh DG, Simpson PC. Knockout of the α1A/C-adrenergic receptor subtype: the α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA 99: 9474–9479, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell A, Watts S. Vascular reactivity of isolated thoracic aorta of the C57BL/6J mouse. J Pharmacol Exp Ther 294: 598–604, 2000. [PubMed] [Google Scholar]
- 44.Sabri A, Pak E, Alcott SA, Wilson BA, Steinberg SF. Coupling function of endogenous α1- and β-adrenergic receptors in mouse cardiomyocytes. Circ Res 86: 1047–1053, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Salomonsson M, Oker M, Kim S, Zhang H, Faber JE, Arendshorst WJ. α1-Adrenoceptor subtypes on rat afferent arterioles assessed by radioligand binding and RT-PCR. Am J Physiol Renal Physiol 281: F172–F178, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Simon P Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19: 1439–1440, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Sitzler G, Zolk O, Laufs U, Paul M, Bohm M. Vascular β-adrenergic receptor adenylyl cyclase system from renin-transgenic hypertensive rats. Hypertension 31: 1157–1165, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Tanoue A, Koba M, Miyawaki S, Koshimizu TA, Hosoda C, Oshikawa S, Tsujimoto G. Role of the α1D-adrenergic receptor in the development of salt-induced hypertension. Hypertension 40: 101–106, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 109: 765–775, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with β2-adrenergic receptors promotes surface expression and functional activity of α1D-adrenergic receptors. J Pharmacol Exp Ther 313: 16–23, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Villalobos-Molina R, Ibarra M. α1-Adrenoceptors mediating contraction in arteries of normotensive and spontaneously hypertensive rats are of the α1D or α1A subtypes. Eur J Pharmacol 298: 257–263, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Villalobos-Molina R, Ibarra M. Vascular α1D-adrenoceptors: are they related to hypertension? Arch Med Res 30: 347–352, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, Edvardsen T, Bjornerheim R, Ahmed MS, Mikkelsen BW, Oie E, Attramadal T, Skomedal T, Smiseth OA, Koch WJ, Larsen TS, Attramadal H. Cardiac-restricted expression of the carboxyl-terminal fragment of GRK3 uncovers distinct functions of GRK3 in regulation of cardiac contractility and growth: GRK3 controls cardiac α1-adrenergic receptor responsiveness. J Biol Chem 283: 10601–10610, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Wiesel P, Mazzolai L, Nussberger J, Pedrazzini T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 29: 1025–1030, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Woodcock EA Roles of α1A- and α1B-adrenoceptors in heart: insights from studies of genetically modified mice. Clin Exp Pharmacol Physiol 34: 884–888, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics 10: 211–215, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto Y, Koike K. Characterization of α1-adrenoceptor-mediated contraction in the mouse thoracic aorta. Eur J Pharmacol 424: 131–140, 2001. [DOI] [PubMed] [Google Scholar]