Abstract
The dehydrins are a class of drought-induced proteins in plants that lack a fixed three-dimensional structure. Their specific molecular action, as well as the reason for their disordered character, is as yet poorly understood. It has been speculated, however, that the dehydrins are tuned to acquire a biologically active structure only under the conditions in which they normally function (i.e. upon dehydration). To test this hypothesis, we here investigate the effect of reduced water content and macromolecular crowding on three dehydrins from Arabidopsis (Arabidopsis thaliana). As a simplistic model for mimicking cellular dehydration, we used polyethylene glycol, glycerol, and sugars that plants naturally employ as compatible solutes (i.e. sucrose and glucose). Macromolecular crowding was induced by the large polysaccharides Ficoll and dextran. The results show that the dehydrins are remarkably stable in their disordered state and are only modestly affected by the solvent alterations. A notable exception is the dehydrin Cor47, which shows a small, intrinsic increase in helical structure at high concentrations of osmolytes. We also examined the effect of phosphorylation but found no evidence that such posttranslational modifications of the dehydrin sequences modulate their structural response to osmolytes and crowding agents. These results suggest that the dehydrins are highly specialized proteins that have evolved to maintain their disordered character under conditions in which unfolded states of several globular proteins would tend to collapse.
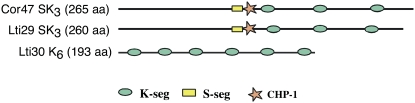
The loss of water from plant cells during drought and freezing triggers a series of adaptation processes. One of these is the production of osmolytes such as Suc, Pro, and betaine (Hasegawa et al., 2000; Wang et al., 2003). As a consequence, the already crowded interior of the cell gets even denser and the physiological processes need to adjust to elevated backgrounds of nonreacting macromolecules and steric confinement. In some cases, the increase in macromolecular density can be radical, going from 300 to 400 g L−1 under ideal growth conditions to >900 g L−1 upon severe desiccation (Ellis, 2001a, 2001b; Bryant et al., 2005). Such cellular crowding not only presents a challenge in terms of modulating diffusion-based chemical reactions (Ellis, 2001a; Minton, 2005a, 2005b, 2006) but will also affect structural integrity. Crowding promotes protein assembly by favoring compact conformations over extended ones, and the reduction in bulk water will directly influence membrane topology and dynamics. The molecular conditions in water-starved plant cells, therefore, are very different from those normally used to characterize biochemical processes in vitro. It has been put forward that in such situations the biological function of proteins could be regulated through crowding-induced conformational changes (Hall, 2006; Hall and Dobson, 2006). Under these extreme conditions, the plant cells respond by expressing a characteristic class of stress proteins, the dehydrins (Close, 1997; Garay-Arroyo et al., 2000; Fig. 1).
Figure 1.
Distribution of the conserved segments in the sequences of the dehydrins Cor47, Lti29, and Lti30 from Arabidopsis. The dehydrins constitute group 2 of the large family of the hydrophilic LEA proteins (Close, 1996; Bartels and Salamini, 2001). The amino acid sequences are as follows: Cor47, MAEEYKNNVPEHETPTVATEESPATTTEVTDRGLFDFLGKKEEEVKPQETTTLESEFDHKAGISEPELAAEHEEVKENKITLLEELGEKTEEDEENKPSVIEKLHRSNSSSSSSSDEEGEEKKEKKKKIVEGEEDKKGLVEKIKEKLPGHHDKTAEDDVPVSTTIPVPVSESVVEHDHPEIKEKLPGYHAKTTEEEVKKEKESDD; Lti29, MAEEYKNTYPEGETPKVATEESSAPEIKERGMFDFLKKKEEVKPQETTTLASEFEHKTGISEPESFVAKHEEEEHKPTLLEGLHQKHEEEEENKPSLLDKLHRSNSSSSSSSDEEGEDGEKPEEEEKKGFMDKIKEKLPGHSKKPEDSGVVNTTPLVETATPIADIPEEKKGFMDKIKEKLPGYHAKTTGEEEKKEKVSD; and Lti30, MNSHQNQTGVQKKGITEKIMEKLPGHHGPTNTGVVHHEKKGMTEKVMEQLPGHHGATGTGGVHHEKKGMTEKVMEQLPGHHGSHQTGTNTTYGTTNTGGVHHEKKSVTEKVMEKLPGHHGSHQTGTNTAYGTNTNVVHHEKKGIAEKIKEGLPGHHGTHKTGTTTSYGNTGVVHHENKST.
The function of dehydrins is as yet unknown. A complicating factor, from a biochemical perspective, is that they seem to lack ordered structure (Lisse et al., 1996; Soulages et al., 2003; Mouillon et al., 2006). Even so, all dehydrins contain, by definition, at least one copy of a highly conserved sequence segment, the K-seg, and may or may not include other conserved sequences called the S-seg, the Y-seg (Close, 1996), and the charged peptide 1 (ChP-1; Mouillon et al., 2006). Since the dehydrins accumulate at very high concentrations inside cells (Bartels and Salamini, 2001), they are unlikely to be signal molecules or conventional enzymes. The role of the dehydrins is probably of a more general nature. For example, they may stabilize membranes, act as chaperones, or by other means buffer the altered solvent properties inside water-stressed cells (Close, 1996; Garay-Arroyo et al., 2000; Boudet et al., 2006). As a clue to how the stress response is orchestrated molecularly, it has been suggested that the functional structure of the dehydrins is induced by their hydration status (Boudet et al., 2006). Accordingly, the disordered appearance of the dehydrins may be converted into active, three-dimensional structures, provided that the conditions inside a drought-stressed plant cell are sufficiently well reproduced. The disordered conformations seen under dilute conditions would then represent the denatured state and, consequently, tell very little about the dehydrin's actual biological function. This idea is supported by the observations that water can modulate ionization potentials, pKA values, and protein-binding potentials (Kornblatt and Kornblatt, 2002). Other classes of disordered proteins have been found to participate in molecular recognition, regulation, and cell signaling (Dunker et al., 2002; Uversky et al., 2005). Function, then, is induced by folding of the disordered protein into a structured state, where the functional groups are placed at the right locations for interactions. Folding can be initiated (or templated) by binding to a biological partner such as other proteins, DNA, RNA, or metals (Dunker et al., 2002; Tompa and Csermely, 2004).
Of particular interest with respect to the dehydrins is that the conformational changes are sometimes conditional (i.e. the protein structure responds to changes in the environment, such as temperature [McNulty et al., 2006a], pH [Tornroth-Horsefield et al., 2006], availability of water [Luo and Baldwin, 1997], or macromolecular crowding [Minton, 2005a, 2005b; Hall, 2006]). Disorder is used to allow the function to be switched on and off (e.g. a 10% change in the concentration of intracellular proteins can lead to changes up to a factor 10 in the activity of molecular regulatory species; Al-Habori, 2001). Computational studies have recently demonstrated that the effect of crowding, despite its underlying complexity, can be simply mimicked by encapsulation of the proteins in spherical pores (Klimov et al., 2002; Cheung and Thirumalai, 2007). This freedom in how the crowding pressure can be applied suggests that simplified experiments in which crowding effects are tested by the addition of large branched polymers, such as Ficoll and dextran, could produce valid approximations of the in vivo condition. In fact, it was recently verified by NMR that the effect of such crowding agents in vitro reproduces the intracellular conditions fairly well (Selenko et al., 2006).
In this study, we examine whether the addition of macromolecular crowding agents in vitro can force the dehydrins to fold. The progressive substitution of water with various polymers is performed as a simple model of how the cellular interior changes upon drought/desiccation. As representative crowding agents, we chose a series of natural and synthetic osmolytes, the effects of which are well characterized in other systems. To distinguish the response of the full-length dehydrins from that of their constituent K-seg and ChP-1 segments, the latter have been analyzed separately in the form of peptides. The results show that both the full-length dehydrins and the isolated K-seg and ChP-1 segments retain their disordered character at extremely high levels of osmolytes. Moreover, the propensity of the dehydrins to undergo unspecific collapse (compare with amorphous glass) appears even lower than that of denatured states of globular proteins. Taken together, these observations suggest that the dehydrin sequence is highly evolved and adapted to remain disordered under conditions of severe dehydration. In this respect, the dehydrins are different from the class of disordered proteins that rely on folding to become functional. The function of the dehydrins, therefore, is likely to lie in the interactions of the conserved segments with their specific biological targets.
RESULTS
Analysis of Structural Transitions Using Circular Dichroism
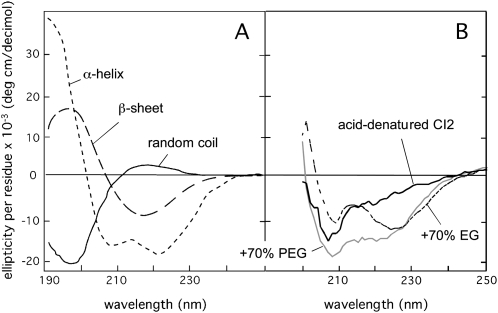
Circular dichroism (CD) is a common spectroscopic method for detecting secondary structure in proteins, where α-helices, β-sheets, and random coils display characteristic spectra at wavelengths of 190 to 250 nm (Fig. 2). Accordingly, the CD spectrum of a typical folded protein is a characteristic structural fingerprint made up by the sum of its constituent secondary-structure elements. In physiological buffer, however, the CD spectra of the dehydrins resemble those of unfolded global proteins (Mouillon et al., 2006; Figs. 2 and 3). This signal differs somewhat from that of the pure random coil because it contains contributions from dynamic, residual secondary-structure and polyproline helices II (Mouillon et al., 2006). In this study, we used this coil-like signal as a reference to test whether a more ordered structure is induced upon the addition of solutes that mimic the solvent conditions experienced inside a living plant cell. For example, the induction of helical structure in the dehydrins upon the addition of trifluoroethylene (TFE; Mouillon et al., 2006) is seen as a characteristic decrease in the CD signal around 222 nm in combination with an increase at around 200 nm (compare with Fig. 2).
Figure 2.
Reference CD spectra of the common types of secondary structure and random coil conformations. A, Pure α-helical structure (dotted line), pure β-sheet structure (dashed line), and random coil (solid line). B, The acid-denatured state of the globular protein CI2 in pure buffer and in 70% EG and PEG. Notably, the structural induction of CI2 by EG and PEG is not along the normal folding pathway but seems to involve nonnative interactions (Silow and Oliveberg, 2003).
Figure 3.
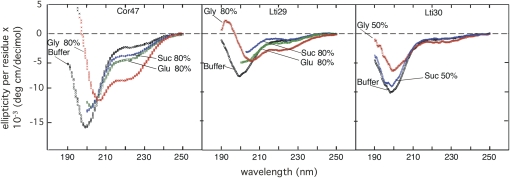
CD signals of Cor47 and Lti29 in the presence of 80% Suc, Glc, and glycerol and of Lti30 in the presence of 50% Suc and glycerol, illustrating differences in responses to the different sugars.
The Natural Stress Solutes Have Little Impact on the Dehydrin Structure
Under conditions of stress, plants are known to produce a variety of compatible solutes, such as betaine (Chen and Murata, 2002), Pro (Yoshiba et al., 1997), and different sugars (Ingram and Bartels, 1996; Hoekstra et al., 2001b). Of particular interest for this study are Suc and Glc, which are naturally produced in response to low temperature and drought (Hoekstra et al., 2001a). To investigate whether increased levels of these sugars contribute to modulating the conformational properties of the dehydrins, we undertook titration experiments (followed by CD) in which the Suc and Glc contents were varied between 0% and 80%. Interestingly, the results suggest that Suc and Glc have only marginal effects on the disordered character of Cor47, Lti29, and Lti30 (Fig. 3). Even at very high concentrations of sugar, the CD signal at 222 nm remains largely the same as in physiological buffer, apart from a small but significant decrease in ellipticity between 210 and 230 nm for Cor47. At shorter wavelengths, the characteristic minimum of the random-coil signal tends to be progressively weakened. Under the extreme solvent conditions used here, however, these spectral changes should be considered relatively modest. The increased CD signal at 200 nm possibly reflects a small increase in helical structure. Such increased helical propensity is expected upon weakening of the hydrogen bonding between water and the protein backbone at high concentrations of solute. Even so, the strength of this signal is too small to imply that the observed effect has any bearing on the physiological function of the dehydrin proteins.
The Effect of Glycerol Follows the Intrinsic α-Helical Propensities of the Polypeptide Chains
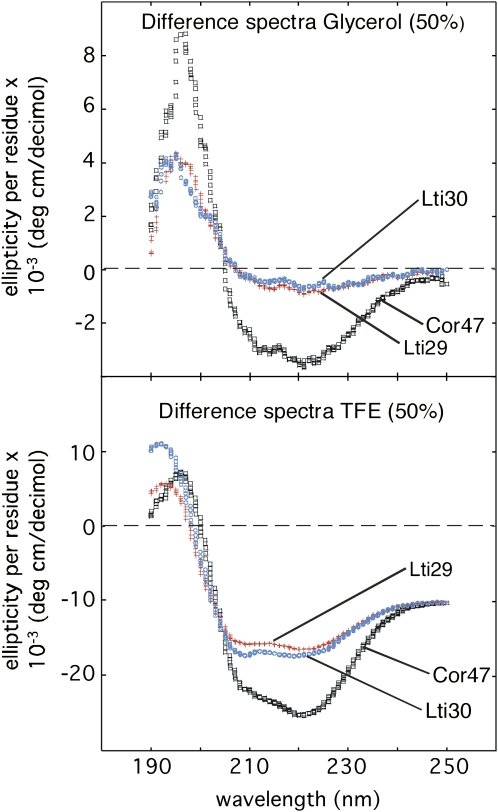
In comparison with the small sugars Suc and Glc, the structural effect of adding glycerol is much more pronounced. Above 50% glycerol, the CD spectra of all proteins in this study indicate a significant increase in the occupancy of α-helical structure (Fig. 3). Moreover, this effect seems to be larger for Cor47 than for Lti29 and Lti30. This result is in good accord with the intrinsic helical propensities of the three proteins. The relative responses of the different dehydrins to additions of glycerol follow precisely that of the helical inducer TFE (Mouillon et al., 2006). In other words, the magnitude of the glycerol response can be correlated to the protein's intrinsic α-helical propensity (i.e. Cor47 > Lti30 = Lti29). To illustrate this correspondence more clearly, in Figure 4 we compare the effect of adding 50% glycerol with that of adding 50% of the strong helical inducer TFE. Although the magnitude of the glycerol response is in general much smaller than that of the TFE response, the features of the difference spectra appear very much the same. Analysis of the different CD spectra using the structural prediction program Dichroweb (Whitmore and Wallace, 2004) shows that Cor47 is 15% α-helical in 50% glycerol, while the corresponding value of Lti29 and Lti30 is 7.5%. Upon comparison with other proteins, it is apparent that this low α-helical propensity matches the background levels of most polypeptide sequences and supports the notion that the extent of residual α-helical structure observed in this study is unlikely to constitute the key to the molecular action of the dehydrins in vivo.
Figure 4.
Top, Difference spectra of Cor47, Lti29, and Lti30 in 50% glycerol. Bottom, Difference spectra of Cor47, Lti29, and Lti30 in 50% of the strong helical inducer TFE. The results indicate that the effects of both glycerol and TFE reflect the intrinsic helical propensity of the dehydrin sequences.
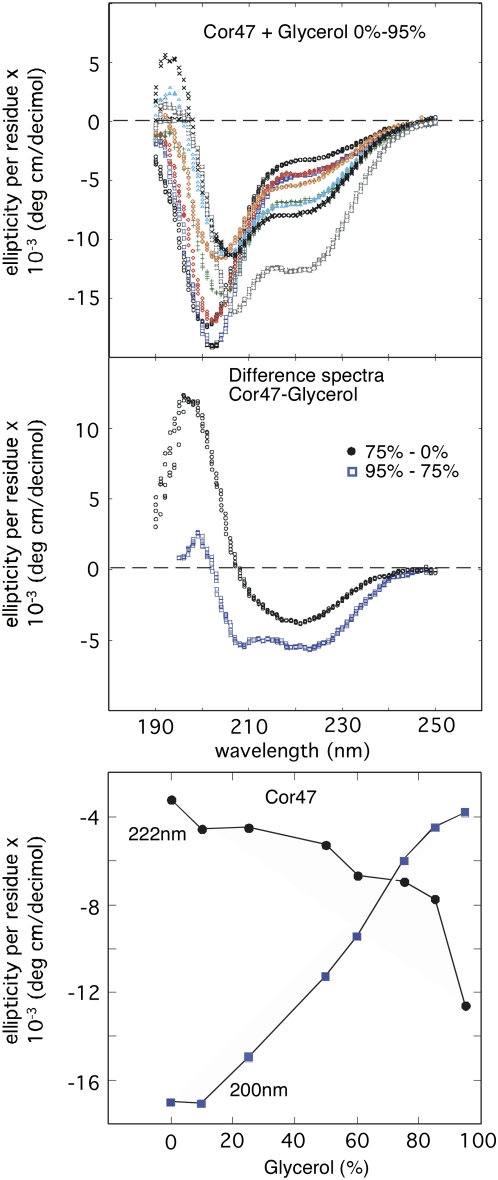
The reason why the α-helical response is higher with glycerol than with Suc and Glc could simply be that the former is a stronger helical inducer (Supplemental Table S1). Even so, we observed that Cor47, which is most sensitive to glycerol, displays a CD change that deviates from an ideal two-state transition into α-helical structure. Upon titration with glycerol, the CD spectrum of Cor47 does not display a single isoelliptic point, as is typical for TFE titrations, but acquires the α-helical signal in two steps (i.e. A ↔ B ↔ C; Fig. 5). First, the CD difference spectra indicate a mixed α-helical structure between 0% and 75% glycerol. Then, the spectrum of Cor47 undergoes a second transition to a more characteristic α-helical signal at >80% glycerol. Apparently, Cor47 first responds to glycerol by adopting low levels of a mixed α-helical structure. Finally, at very high glycerol concentrations, this mixed structure is converted to more purely α-helical structures (Fig. 5). These α-helical structures could first form locally and then extend to cover a larger part of the sequence. Analysis using Dichroweb (Whitmore and Wallace, 2004) shows 22% helical structures at 75% glycerol and 48% helical structures at 95% glycerol. Thus, by quantitative comparison, the results suggest that the degree of α-helical structure induced by 95% glycerol is similar to that in 25% TFE (Mouillon et al., 2006). It should also be noted that at 25% TFE the level of α-helical structure of Cor47, as analyzed using CDPro, levels out at a maximum value of 47% (Mouillon et al., 2006). Therefore, Cor47 is likely to acquire a significant content of local structure in 95% glycerol.
Figure 5.
Top, CD spectra of the glycerol titration of Cor47. The spectra show from top at 220 nm, 0%, 10%, 25%, 50%, 60%, 75%, and 95% glycerol. Middle, CD difference spectra showing that the structural induction of Cor47 with glycerol occurs in two steps. Between 0% and 75%, glycerol Cor47 displays a mix of β-sheet and α-helices, while above 75%, it displays mainly α-helices. Bottom, Changes in the CD signal at 200 and 222 nm upon titration of Cor47 with glycerol, illustrating the transitions to mixed and pure α-helical structures, respectively.
The corresponding signal for Lti29 and Lti30 is much smaller. This indicates that the sensitivity to glycerol is a specific feature of the Cor47 sequence and not a common, functional property of the dehydrin proteins as a group.
Structural Collapse in the Presence of Polyethylene Glycol: Comparison with the Unfolded States of Globular Proteins
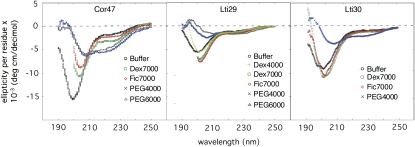
The tendency of the polypeptide chain to collapse into compact states at high concentrations of osmolytes such as glycerol is not unique to Cor47 but has generally been observed for small peptides (Venkatesu et al., 2007) and denatured states of globular proteins (Fig. 2; Foord and Leatherbarrow, 1998; Silow and Oliveberg, 2003). If the pressure to minimize the surface area is sufficiently high, any unfolded polypeptide is expected to collapse into compact forms that need not always resemble the native structures (Bryngelson and Thirumalai, 1996). The ability to resist such unspecific coil collapse under physiological conditions is a key feature that distinguishes natural proteins from randomly assembled polypeptides (Bryngelson and Thirumalai, 1996). This property renders the denatured states of natural proteins flexible and facilitates their folding into unique native structures. In an attempt to benchmark the collapse propensity of the dehydrin proteins along such a general scale, we have monitored the effect of adding polyethylene glycol (PEG), following the conditions used in protein-folding studies. Again, the structural response was highest for Cor47, followed by Lti30 (Fig. 6). Least affected was Lti29. As the effects of PEG 4,000 and PEG 6,000 resemble that of glycerol, the mode of action of these osmolytes is most likely similar. Analysis of the different PEG spectra using Dichroweb reveals that the induced structure is a mix of β-sheets and α-helices (14% helical in 50% PEG 6,000). It is interesting, however, that the susceptibility of the dehydrins to change their structure in the presence of osmolytes generally seems lower than for denatured states of small globular proteins (compare with Figs. 2 and 6). Although the globular proteins typically adopt their folded states in highly concerted two-state transitions (i.e. without populating intermediates), their denatured states have been observed to become trapped in compact, sterically restricted conformations already at 30% PEG or ethylene glycol (EG; Qu et al., 1998; Silow and Oliveberg, 2003). In the case of the archetypical two-state protein CI2, the acid-denatured state shows striking changes in the CD spectrum at 70% PEG (Fig. 2; Silow and Oliveberg, 2003). Comparable effects are only seen with Cor47, while the impact on the CD spectra of Lti29 and Lti30 is less pronounced. Consistently, the dehydrins also show very limited response to sulfate, which is another well-known misfolding and aggregation agent for globular proteins (Otzen and Oliveberg, 1999). Therefore, when it comes to resisting unspecific collapse, it is apparent that the dehydrins are equally, if not more, robust than the unfolded states of globular proteins.
Figure 6.
Crowding of Cor47, Lti29, and Lti30 with polymers. The concentration of polymers is 50%.
The Response of the Dehydrins to the Crowding Agents Ficoll and Dextran
The preferred way to examine the effect of macromolecular crowding would be to subject the dehydrins to high concentrations of other proteins, RNA, and colloidal particles. In vivo, the total concentration of proteins and RNA is in excess of 300 mg mL−1 (Zimmerman and Trach, 1991). An intrinsic problem with such experiments, however, is that the spectroscopic handle of the dehydrin structure, in this case the CD signal, would disappear against the much larger background of the cosolutes. As an alternative, we used the large polysaccharides Ficoll (70 kD) and dextran (40 and 70 kD). Interestingly, the presence of these crowding agents showed no appreciable effect on the dehydrin proteins. At 50% Ficoll or dextran, the CD spectra of Lti29 and Lti30 were virtually identical to those in pure buffer, while the spectrum of Cor47 showed an increase in the 200-nm region similar to that in Glc and Suc (Fig. 6). Although the changes in the lower wavelengths of the Cor47 spectrum are difficult to rationalize structurally (Mouillon et al., 2006), the results on the whole underline the dehydrins' resistance to altering their disordered character in response to cosolute exposure. Therefore, the disordered character of these proteins could be a key requirement for their function, and this property seems to be maintained in the crowded interior in the dehydrated plant cell.
Combinations of Sugars and Osmolytes
Although tests of the pure individual osmolytes and crowding agents are required for experimental stringency, these experiments need not represent the complex conditions inside stressed cells, where a multitude of cosolutes act in concert. To examine whether there are any synergistic effects of mixing sugars, osmolytes, and crowding agents, we also screened the response of the dehydrins to a series of mixtures. As a base, we used 25% glycerol and added to this an additional 25% of the other compounds. Notably, these systematic mixtures did not contribute to enhancing or changing the effect on the dehydrin CD spectra: in all cases, the observed effect was the combination of the signals from the isolated compounds. To further rule out the possibility of a delayed response to the solvent perturbations, the samples were rerun after 24 h but without any detectable difference in the CD spectra (data not shown). These findings are in line with the recent conclusions by Holthauzen and Bolen (2007).
The Conserved Segments Behave Approximately Like the Full-Length Proteins
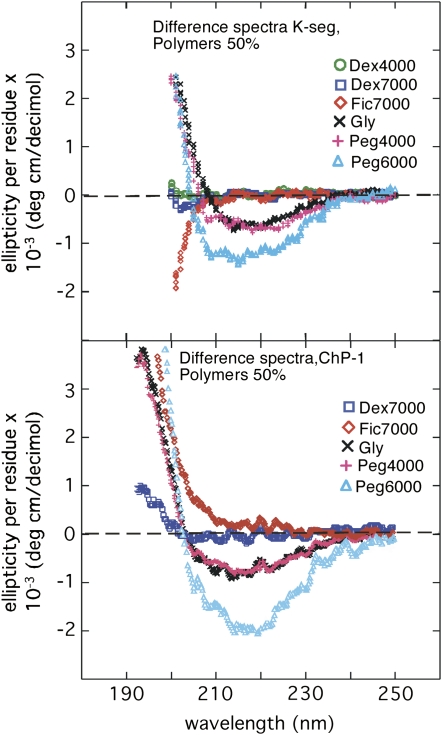
Even though the response of the full-length proteins to solvent additives is generally small, it is possible that this response stems from a strong structural effect in a small part of the sequence. The prime candidates for such local structure formation would then be the sequence regions containing the conserved segments. These are the K-seg (EKKGIMDKIKEKLPG) and ChP-1 (EEGEDGEKKKKEKKKKI), in other words, the charged part following the Ser-rich segment found in Cor47 and Lti29 (i.e. in the SKn type of dehydrins; compare with Fig. 1). The functions of these characteristic and repeatedly occurring sequence stretches are still unknown. In partial contrast to the full-length dehydrins, peptides of the K-seg and ChP-1 show the most pronounced structural response to PEG 6,000 and not to glycerol (Fig. 7). At 50% PEG 6,000, structural prediction analysis using Dichroweb (Whitmore and Wallace, 2004) suggests an α-helical content of 9% for K-seg and 11% for ChP-1. By comparison, the effect of PEG 4,000 is substantially smaller than that of PEG 6,000 and similar to the structural response induced by glycerol.
Figure 7.
CD difference spectra of the conserved segments of the dehydrins, showing the effect of adding 50% polymers. Top, The K-seg (EKKGIMDKIKEKLPG) is present in all dehydrins. Bottom, The ChP-1 segment (EEGEDGEKKKKEKKKKI) is present in Cor47 and Lti29.
The different effects of PEG 6,000 and PEG 4,000 are interesting, given that the PEGs are substantially larger than the peptide fragments and the full-length dehydrins and would, accordingly, be expected to affect these in an equivalent way. Ficoll and dextran are also considerably larger than either peptide but give as low a response as with the full-length dehydrins. Upon titration with glycerol, the K-seg and ChP-1 both show an approximately linear decrease of the CD signal at 222 nm. Therefore, K-seg and ChP-1 behave very much like the full-length proteins Lti29 and Lti30 and lack the characteristic late transition of Cor47 at 95% glycerol (data not shown).
Phosphorylation Has Only a Marginal Effect on the Dehydrin Structures
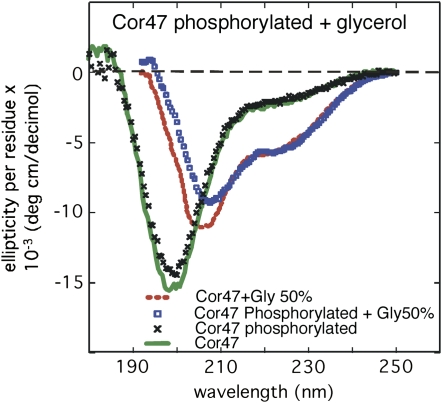
Recombinantly expressed dehydrins are not phosphorylated when purified from Escherichia coli (data not shown). However, dehydrins are known to undergo phosphorylation both in vivo and in vitro (Jiang and Wang, 2004; Alsheikh et al., 2005; Brini et al., 2006; Rohrig et al., 2006). To investigate the structural impact of phosphorylation, we treated the dehydrins with casein kinase II. Both Cor47 and Lti29, but not Lti30, became phosphorylated. Such selective phosphorylation of the different dehydrins is in good agreement with previous reports (Alsheikh et al., 2005). These results show that phosphorylation of Lti29 has no detectable effect on the CD spectra under any of the solvent conditions tested in this study (data not shown). In the presence of 50% glycerol, however, phosphorylation of Cor47 produces a small decrease in the coil content, indicated by the CD spectrum below 210 nm (Fig. 8). Notably, this signal cannot be seen in the presence of Glc or PEG 4,000, suggesting that the structural relevance is after all very small (data not shown). The effect of phosphorylation on the dehydrin structure, therefore, is very small and does not significantly enhance the response to osmolytes or crowding agents in vitro.
Figure 8.
CD spectra of phosphorylated Cor47 in pure buffer and in 50% glycerol.
DISCUSSION
Crowding Has No Observable Role in Modulating the Dehydrin Structures
How the molecular functions of proteins and membranes are maintained in organisms during environmental stress is a poorly understood area of biology that relates to the detailed interplay between solvent and solute molecules. The latter include suprastructures of lipids, proteins, and carbohydrates. In plants, drought can reduce the water content to only a fraction of the normal levels, often accompanied by compensating production of osmolytes and changes in protein composition. The benefit of such stress-induced adjustments of the intracellular conditions is to safeguard biological viability. At the molecular level, however, not only is it challenging to reproduce this complex response in vitro but the response is also challenging to rationalize mechanistically: the interior of cells cannot yet be treated exactly as in vivo, and simplifications are needed. In this study of the dehydrin proteins, one such simplification is to parameterize the experimental conditions in terms of macromolecular crowding or excluded volume effects. As the water content of the cell is decreased, the background of other molecules is enriched, leading to a geometric restriction of the available space for polypeptide chains. Typically, proteins function under conditions in which the solute concentration is around 400 g L−1 (Zimmerman and Minton, 1993), and in severely dehydrated plant cells this number can be substantially higher (Bartels and Salamini, 2001). Such increased background of crowding molecules (i.e. the osmolytes and all other solvent components taken together) is expected to have an important impact on the cellular processes (Ellis, 2001a, 2001b). By simply competing for volume, macromolecular crowding favors compact states of the polypeptide chain and increases the stability of native proteins, provided that no competing misfolded conformations are produced. Accompanying this general stability effect, crowding also increases the occupancy of compact denatured species, which, eventually, leads to a retardation of the protein-folding rates due to increased reconfiguration times. As the normally flexible and dynamic polypeptide chain gets entangled in compact conformations, its internal movements become energetically more cumbersome (Bryngelson and Thirumalai, 1996; Oliveberg and Wolynes, 2005). It has been put forward that such collapse/expansion of certain inert biopolymers could actually be used as a means to regulate the functions of proteins in biological environments via crowding (Hall, 2006; Hall and Dobson, 2006).
The effect of macromolecular crowding on the protein structure, therefore, is expected to resemble that of small stabilizing agents such as sodium sulfate (Na2SO4). However, the efficiency of macromolecular crowding in promoting protein stability is relatively modest and is substantially smaller than that of Na2SO4. The equilibrium constant for RNAse T1 was only shifted 7.5-fold toward the native state in 400 mg mL−1 dextran 70 (Qu and Bolen, 2002), while the corresponding effect of Na2SO4 on the protein L23 is several times larger (Hedberg and Oliveberg, 2004). Consistent with the earlier observation that Na2SO4 has no appreciable impact on the dehydrin structures (Mouillon et al., 2006), we could not discern any compelling effect upon crowding by the large branched sugars Ficoll and dextran (Fig. 6). The only outcome is a small change in the low-wavelength region of the CD spectra. This change could possibly reflect a relaxation of PII helices toward more flexible coil conformations. By comparison, induction of a classic secondary structure, such as α-helices or β-sheets, would lead to a reduction in the 200-nm signal with a simultaneous increase in the minimum around 220 nm (compare with Fig. 2). However, it is necessary to consider also the size of the tested crowding agent in relation to that of the dehydrins. At a first approximation, a small crowding agent is expected to get closer to the protein and, hence, to occupy volume more efficiently compared with a large crowding agent. Even so, the dehydrin structures are equally indifferent to the smaller sugars Glc and Suc (Figs. 3 and 4).
We could not distinguish any correspondence between changes in CD spectra and the size of any of the added solutes in this study. The disordered nature of the dehydrin polypeptide seems to persist also when the available space is geometrically restricted. A plausible explanation for this result is that the dehydrins do not easily adapt compact conformations, either in the form of uniquely defined folded states, as has been observed for several other disordered proteins (Dawson et al., 2003; Love et al., 2004; Fink, 2005), or in the form of unspecifically collapsed conformations that are sometimes observed in the denatured states of globular proteins (Lattman et al., 1994; Otzen and Oliveberg, 2002; Ittah et al., 2004). The behavior is in good agreement with earlier reports on the disordered c-Fos and p27Kip1 proteins, in which crowding with differently sized dextrans (9, 37.5, or 77 kD) and Ficoll 70 at concentrations of up to 250 g L−1 was not sufficient to induce an ordered structure (Flaugh and Lumb, 2001). The dehydrins resemble also the Parkinson-related α-synuclein that remains disordered in the crowded interior of E. coli cells and at high concentrations of bovine serum albumin (McNulty et al., 2006b). In contrast, the transcription factor regulator FlgM partly gains structure under the corresponding conditions (Dedmon et al., 2002), and FlgM also gains structure upon binding to its biological target (Daughdrill et al., 1997). These differences have been taken to exemplify two classes of disordered proteins: those that are functionally tuned to remain disordered in the crowded interior of the cell (Flaugh and Lumb, 2001) and those that are not. According to the data in Figures 3 to 8, the dehydrins belong to the former category. Taken together, the effect of macromolecular crowding on disordered protein conformations seems to be variable and needs to be tested in each separate case. A brief summary of the crowding effects on other disordered proteins and denatured globular proteins is given in Supplemental Table S1. Another effect of water loss is increased ionic strength. We demonstrated earlier, however, that increased ionic strength or elevated levels of metal ions has no appreciable impact on the dehydrin structure in vitro (Mouillon et al., 2006).
Preferential Protein Solute Exclusion Increases the Sampling of the Local α-Helical Structure
The conformational transitions underlying the altered CD signals of Cor47 in the presence of glycerol and PEGs, therefore, are likely to have a molecular origin different from pure crowding. From a functional perspective, it is reasonable to examine whether these molecular factors are somehow coupled to changes in the accessibility of water. For example, they may be manifested in altered structures of the protein hydration shells. However, such water-mediated effects are intrinsically difficult to mechanistically rationalize because of the complexity of aqueous solutions. A simplified view is offered by weak interaction models. These models assume that the solute molecules, typically osmolytes, are either accumulated or excluded from the protein-solvent interface. In the event that the osmolyte is preferentially attracted to the protein surface, this will favor extended conformations and promote destabilization according to mass action (Schellman, 2002). Examples of such destabilizing osmolytes are the denaturants urea and guanidium chloride. Conversely, if the osmolyte is preferentially excluded from the protein surface, this will lead to minimization of the protein-solvent interface area and protein stabilization (Bolen and Baskakov, 2001). Such stabilizing, or protective, osmolytes are sugars, free amino acids, and glycerol. The reason for the “affinity differences” between the osmolytes seems partly related to the degree to which they can form favorable polar interactions with the polypeptide backbone (Street et al., 2006). Computational (Street et al., 2006) and experimental (Qu and Bolen, 2002) analyses suggest that the order of stabilizing capacity of the osmolytes used in this study is Suc > sorbitol > Pro > glycerol. From this perspective, it is interesting that the osmolyte that most efficiently perturbed the CD spectra of the dehydrin Cor47 is glycerol (Fig. 5). The effect of 80% Suc, on the other hand, is still relatively modest. An explanation for this reversed order could lie in the somewhat unusual amino acid composition of the dehydrin sequences.
The computational ranking of the osmolyte effect is obtained from backbone interactions alone (Street et al., 2006), which is in good accord with the stability effect on RNAse T1 (Qu and Bolen, 2002) with a hydrophobic side chain content typical of globular proteins (compare with pdf structure 1bu4 in the RCSB Protein Data Bank). In contrast, the dehydrin sequences contain a relatively high proportion of charged and polar side chains, presenting a higher degree of hydrogen-bonding opportunities with the surrounding solution molecules. This peculiarity could also explain why α-synuclein, in contrast to the dehydrins, collapses upon addition of Glc (Morar et al., 2001): α-synuclein contains approximately 40% hydrophobic residues, while the dehydrins contain just 15% (Lti30) to 30% (Cor47 and Lti29).
Even so, and regardless of the mechanistic origin of the accentuated glycerol effect of the dehydrins, it is apparent from the CD data that the structural impact of adding osmolytes is only small at physiologically relevant concentrations (Figs. 3 and 5). The same is true for the fragments of their conserved segments (Fig. 7). The glycerol effect is not clearly manifested unless the glycerol content is increased above 30%, a level that is likely to greatly exceed the physiological concentrations. By comparison, the highest reported level of Suc in vivo is 40% of the dry weight, and this in a resurrection plant that more or less tolerates complete desiccation (Ingram and Bartels, 1996). Moreover, the structural transition observed at extreme concentrations of glycerol corresponds mainly to an increased sampling of the α-helical structure, which is in good accord with the intrinsic α-helical propensity of the different dehydrin proteins (Fig. 1). The largest effect was observed for Cor47, followed by Lti29 and Lti30, which precisely matched the gain of the α-helical structure in TFE titrations (Mouillon et al., 2006). Consistently, a similar structural response was observed upon the addition of PEG 4,000 and PEG 6,000. These polymers are well-known protein stabilizers (Kornblatt and Kornblatt, 2002; Kozer and Schreiber, 2004; Ren et al., 2006) that have also been observed to unspecifically collapse the denatured states of globular proteins (Silow and Oliveberg, 2003). On this basis, we conclude that the exertion of pressure to minimize the solvent-accessible surface area of the dehydrin chains has no appreciable effects on their tertiary structures. They generally remain disordered. The effect on the local α-helical content merely follows the intrinsic α-helical propensity of the polypeptide chains. The latter, however, is more likely to reflect the generic properties of the polypeptide chain than specific function traits.
Possible Interactions between Dehydrins and Lipid Membranes
A frequently proposed function of dehydrins and other LEA proteins is as membrane stabilizers. In essence, the dehydrins would interact with membranes to modulate their topology and phase transitions under stress, and in this process the dehydrins could also gain ordered structure. This idea is supported by several findings of interactions between lipid vesicles and dehydrins and other LEA proteins (Soulages et al., 2002, 2003; Koag et al., 2003; Tolleter et al., 2007; Kovacs et al., 2008). In some cases, as with the maize (Zea mays) dehydrin DHN1, the membrane interaction leads to conformational changes in the protein's secondary structure (i.e. an increase in the α-helical content; Koag et al., 2003). However, it is not clear to what extent these effects can be transferred to other members of the protein family, as, in contrast, the interaction between Lti29 and negatively charged lipids did not result in any conformational changes (Kovacs et al., 2008). Also, the presence of lipids failed to induce any structural effect on the soybean (Glycine max) LEA protein GmD-19 and dehydrin GmDHN1 (Soulages et al., 2002, 2003). Although the involvement of the dehydrins in safeguarding membrane structure during stress is indeed an attractive possibility, its generality remains to be established.
Phosphorylation Does Not Generate Any Significant Structural Changes of Dehydrins
Phosphorylation is a posttranslational modification that is essential for the regulation of cellular functions. It was recently shown that most phosphorylation sites, 86%, are within disordered regions of proteins (Collins et al., 2008). Even so, there are few studies on the effect of phosphorylation on the structure of disordered proteins. Of particular interest for this study is the fact that phosphorylation of the dehydrins occurs in vivo and has been reported to modulate the coordination of calcium ions (Jiang and Wang, 2004; Alsheikh et al., 2005; Brini et al., 2006; Rohrig et al., 2006). In this study, only Cor47 and Lti29 could be phosphorylated by casein kinase II. The result of this phosphorylation, however, had no significant bearing on the structural behavior of Cor47 and Lti29. Recent NMR studies of α-synuclein have shown that phosphorylation at Ser-129 by casein kinase II increases the disordered character of the protein. It was further shown that this structural alteration increased the protein's tendency to self-associate (Sasakawa et al., 2007). As seen in Figure 8, the structure of Cor47 is left unaltered upon phosphorylation and also shows no tendency to aggregate. Crowding by glycerol makes no difference. Even though Cor47 and Lti29 seem to be structurally unchanged, it is important to note that phosphorylation can still play a key role as a regulatory factor for the interaction with other cellular targets.
CONCLUSION
From a physiological standpoint, the separate tests of crowding agents and osmolytes constitute a fairly crude representation of the intracellular conditions in which spatial confinement and the presence of complex mixtures of background molecules act in concert on the dehydrin structures. However, we failed to observe any synergistic structural effects in mixtures of glycerol and the other solutes used in this study, lending further support to the idea that the dehydrin structures are generally insensitive to changing solvent conditions. Based on previous results, the structural insensitivity also includes changes of ionic strength and levels of metal ions (Mouillon et al., 2006). This suggests that the dehydrin sequences are evolved to remain flexible also under severe stress conditions and that this property is an integral requirement of their physiological function. Possibly, the high content of charged and polar residues of the dehydrins effectively prevents the structural collapse due to the accompanying desolvation penalties of ionic moieties and hydrogen bond partners. Such transitions into compact glass-like species are otherwise characteristic of random polymers (Bryngelson and Thirumalai, 1996) and to some extent also of denatured states of globular proteins (Silow and Oliveberg, 2003). In both instances, the affected sequences generally contain higher fractions of hydrophobic side chains than observed for the dehydrins.
Additional indications that the dehydrins are tuned to remain flexible under in vivo conditions come from their high solubility and resistance to precipitation (Mouillon et al., 2006). As a contrasting example, α-synuclein readily assembles in ordered fibrillar aggregates at elevated protein concentrations or upon the addition of PEG/osmolytes (Goers et al., 2003; Fink, 2006). In discussions about the biological function of the dehydrins, it has been pointed out that their unfolded nature would effectively maximize the coordination of water. According to NMR experiments, this water is more tightly bound than in other disordered proteins (Bokor et al., 2005). Some of the water seems tied up in bridging interactions within PII helices (Mouillon et al., 2006). Upon removal of the coordinated water by evaporation, other proteins in the LEA family have been observed to undergo structural rearrangements (Wolkers et al., 2001; Goyal et al., 2003, 2005). For example, the LEA protein AavLea1 increases its content of α-helical structure (Goyal et al., 2003). Could the dehydrins then act as water reservoirs that expel coordinated water to the surrounding medium (Boudet et al., 2006)? Considering the finding that the in vivo concentration of dehydrins does not seem to exceed 2% to 4% of cytoplasmic proteins, however, the benefit of such water expulsion is likely to be marginal (Roberts et al., 1993; Ceccardi et al., 1994; Close, 1996), at least in moderate drought. More likely, the molecular action of the dehydrins is more specific. One possibility is that the dehydrins provide structural support, either spatially by cross-linking with other macromolecular constituents (Abu-Abied et al., 2006) or laterally by adhering to membrane surfaces (Tolleter et al., 2007). Desiccation of the cell will not only alter the molecular composition of the aqueous compartments of the cell but also distort its physical dimensions, including the shape and proximity of membrane structures.
It is easy to envisage that such global alterations could have a pronounced effect on the colloidal processes, for example by promoting phase transitions, topological frustration, and altered dynamics of membrane fusion and budding. The role of the dehydrins may be to intervene with these processes by filling out, and perhaps topologically organizing, crevices in bulging membranes and thereby safeguarding their structural integrity. Alternatively, the dehydrins could support macromolecular structures of proteins, carbohydrates, or nucleotides. In any case, the elements of recognition with the cellular target are likely to be the conserved segments. The remarkable capacity of the dehydrins to remain coil like suggests that the high flexibility of the sequence regions connecting these segments is an important part of their function (Fig. 1): the conserved segments are organized as “hooks on a string” (Mouillon et al., 2006). Notably, this arrangement not only restricts the spatial location of the conserved elements but also increases their local concentration.
MATERIALS AND METHODS
Expression and Heat Fractionation
Expression, purification, and identification of the recombinant Arabidopsis (Arabidopsis thaliana) dehydrin proteins were as described previously (Svensson et al., 2000). Glycerol stocks of the different Escherichia coli strains were made, and 200 μL was spread on Luria agar plates with 150 μg of ampicillin. The plates were kept at 37°C and grown overnight. The cells were suspended and added to 1 L of Luria-Bertani medium containing 50 μg mL−1 ampicillin. Expression was induced at an optical density of 0.3 by adding isopropyl β-d-thiogalactopyranoside to a final concentration of 1 mm. The suspensions were kept at 37°C until grown to an optical density of 0.8 to 1.0 (i.e. 2–4 h). Cells were harvested by centrifugation at 4,000g for 45 min, and the pellet was stored at −20°C. The thawed cells from 1-L cultures were resuspended in 25 mL of 20 mm Na2HPO4, pH 7.2, and 150 mm NaCl. To this was added 1 mm (final concentration) phenylmethylsulfonyl fluoride and 0.1 mg mL−1 lysosyme, and the suspension was left for 30 min on ice. Lysated cells were sonicated six times for 15 s each and centrifuged at 9,000g for 30 min. The supernatants were placed in a water bath at 70°C for 20 min. To precipitate heat-denatured proteins, the sample was centrifuged for 30 min at 9,000g, and the supernatant was stored at −80°C.
Purification of Dehydrins
Purification of dehydrins by immobilized metal ion affinity chromatography and the following step by ion-exchange chromatography was according to Svensson et al. (2000). The supernatants from heat precipitation were diluted 1:2 with 20 mm NaHPO4, pH 7.2, 1.85 m NaCl, and 1 mm phenylmethylsulfonyl fluoride. The samples were loaded on a 5-mL HiTrap IDA-Sepharose column (Pharmacia) connected to a FPLC system (Pharmacia) and charged with 2 mL of 3 mg mL−1 CuSO4. Absorbance was read at 280 nm. Before applying the sample, five volumes of 20 mm Na2HPO4, pH 7.2, and 1.0 m NaCl were used to equilibrate the column. The same buffer (40 volumes) was used to wash off unbound sample from the column. Fractions of 5 mL were collected for analysis during the whole run. Elution was performed with 2 m NH4Cl in 20 mm Na2HPO4, pH 7.2, and 1.0 m NaCl in one step. The column was then equilibrated with 10 volumes of 20 mm Na2HPO4, pH 7.2, followed by elution of the copper with 10 mm EDTA in 20 mm Na2HPO4, pH 7.2. Precipitation of protein was done with 80% (NH4)2SO4, and proteins were collected by centrifugation at 18,000g for 30 min. The different dehydrins were resuspended in 2 mL of the following buffers: Lti29 and Cor47 in 20 mm BisTris, pH 6.0, Rab18 in 20 mm Tris-HCl, pH 8.0, and Lti30 in 50 mm Gly, pH 9.0. The dehydrins were desalted on a PD-10 column (Pharmacia) by the resuspension buffers. The achieved 3-mL fractions were put on an anion-exchange column (1-mL Mono Q HR 5/5; Pharmacia) in the case of Cor47 and Lti29 and on a cation-exchange column (1-mL Mono S HR 5/5; Pharmacia) connected to an FPLC system in the case of Lti30, and absorbance was read at 280 nm. The columns were equilibrated with the resuspension buffers and elution by a NaCl gradient from 0 to 0.5 m in the respective buffer over 30 volumes. Fractions of 0.5 mL were collected during the runs for analysis. The purity was tested by SDS-PAGE, and without exception the purified material was run as untreated dehydrins from crude cell extracts (data not shown). An extensive identification of the recombinant dehydrins has been published by Svensson et al. (2000).
Analysis by CD
CD measurements were carried out using a JASCO J-810 spectropolarimeter, and the results are presented as mean ellipticity per residue. The scan rate was 20 nm min−1 at a band pass of 0.2 nm, with 20 mdeg sensitivity. All samples were mixed 1 h prior to the CD analysis and centrifuged at 12,000g for 2 min before filling the cuvettes. Protein concentration was 1 mg mL−1 in 20 mm MES (0.2-mm cuvette). Unless stated otherwise, all runs were performed at 25°C.
Preparation of Samples
Unless otherwise stated, concentrations are given as percentage referring to grams per liter. Dextrans (average molecular mass of 40 or 70 kD; Sigma), Ficolls (average molecular mass of 70 kD; Sigma), and PEG (average molecular mass of 4,000 or 6,000 D; Sigma) were used in concentrations of up to 500 to 800 g L−1. All osmolytes, sugars, and crowding agents were titrated in steps of 10% to 25%. Each titration series was repeated at least twice. The exception was Lti30, for which the titration steps were 15% to 25% and titration ended at 50% because of the low amount of high-purity Lti30 protein.
Phosphorylation of Dehydrins
The dehydrins were phosphorylated by 5.4 units mL−1 casein kinase II (Sigma) in 1 mm ATP, 20 mm Tris-HCl, 50 mm KCl, and 10 mm MgCl2, pH 7.5, for 10 h. The phosphorylation status of the dehydrins was tested by Pro-Q Diamond Phosphoprotein Gel stain (Invitrogen) on 15% Tris-HCl gels (Bio-Rad), and the background levels of protein were measured by SYPRO Ruby Gel stain (Invitrogen). Stained gels were visualized with an FLA-3000G (Fuji Photo Film) and a UV transilluminator. The phosphorylation status was measured on three different batches of dehydrins, with identical results. For the structural analysis, the casein kinase II was separated from the dehydrins by precipitation at 70°C, followed by centrifugation. The effect of solvent additives on the phosphorylated dehydrins was measured as described above.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers P42758, P42759, and CAA48178.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phosphorylation of Lti29 and Cor47 by casein kinase II.
Supplemental Figure S2. CD spectra of phosphorylated Lti29 in the presence of 50% glycerol.
Supplemental Table S1. Effects of various crowding agents on intrinsically disordered proteins and denatured globular proteins.
Supplementary Material
Acknowledgments
We are most grateful to Professor Mikael Oliveberg (Stockholm University) for the data regarding CI2 and for helpful discussions.
This work was supported by the Swedish Research Council for the Environment, Agricultural Sciences, and Spatial Planning (P.H.), the Carl Tryggers Foundation (P.H.), and the Lawski Foundation (S.K.E.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Pia Harryson (pia.harryson@dbb.su.se).
The online version of this article contains Web-only data.
References
- Abu-Abied M, Golomb L, Belausov E, Huang S, Geiger B, Kam Z, Staiger CJ, Sadot E (2006) Identification of plant cytoskeleton-interacting proteins by screening for actin stress fiber association in mammalian fibroblasts. Plant J 48 367–379 [DOI] [PubMed] [Google Scholar]
- Al-Habori M (2001) Macromolecular crowding and its role as intracellular signalling of cell volume regulation. Int J Biochem Cell Biol 33 844–864 [DOI] [PubMed] [Google Scholar]
- Alsheikh MK, Svensson JT, Randall SK (2005) Phosphorylation regulated ion-binding is a property shared by the acidic subclass dehydrins. Plant Cell Environ 28 1114–1122 [Google Scholar]
- Bartels D, Salamini F (2001) Desiccation tolerance in the resurrection plant Craterostigma plantagineum: a contribution to the study of drought tolerance at the molecular level. Plant Physiol 127 1346–1353 [PMC free article] [PubMed] [Google Scholar]
- Bokor M, Csizmok V, Kovacs D, Banki P, Friedrich P, Tompa P, Tompa K (2005) NMR relaxation studies on the hydrate layer of intrinsically unstructured proteins. Biophys J 88 2030–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen DW, Baskakov IV (2001) The osmophobic effect: natural selection of a thermodynamic force in protein folding. J Mol Biol 310 955–963 [DOI] [PubMed] [Google Scholar]
- Boudet J, Buitink J, Hoekstra FA, Rogniaux H, Larre C, Satour P, Leprince O (2006) Comparative analysis of the heat stable proteome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiol 140 1418–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini F, Hanin M, Lumbreras V, Irar S, Pages M, Masmoudi K (2006) Functional characterization of DHN-5, a dehydrin showing a differential phosphorylation pattern in two Tunisian durum wheat (Triticum durum Desf.) varieties with marked difference in salt and drought tolerance. Plant Sci 172 20–28 [Google Scholar]
- Bryant JE, Lecomte JT, Lee AL, Young GB, Pielak GJ (2005) Protein dynamics in living cells. Biochemistry 44 9275–9279 [DOI] [PubMed] [Google Scholar]
- Bryngelson JD, Thirumalai D (1996) Internal constraints induce localization in an isolated polymer molecule. Phys Rev Lett 76 542–545 [DOI] [PubMed] [Google Scholar]
- Ceccardi TL, Meyer NC, Close TJ (1994) Purification of a maize dehydrin. Protein Expr Purif 5 266–269 [DOI] [PubMed] [Google Scholar]
- Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5 250–257 [DOI] [PubMed] [Google Scholar]
- Cheung MS, Thirumalai D (2007) Effects of crowding and confinement on the structures of the transition state ensemble in proteins. J Phys Chem B 111 8250–8257 [DOI] [PubMed] [Google Scholar]
- Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97 795–803 [Google Scholar]
- Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol Plant 100 291–296 [Google Scholar]
- Collins MO, Yu L, Campuzano I, Grant SG, Choudhary JS (2008) Phosphoproteomic analysis of the mouse brain cytosol reveals a predominance of protein phosphorylation in regions of intrinsic sequence disorder. Mol Cell Proteomics 7 1331–1348 [DOI] [PubMed] [Google Scholar]
- Daughdrill GW, Chadsey MS, Karlinsey JE, Hughes KT, Dahlquist FW (1997) The C-terminal half of the anti-sigma factor, FlgM, becomes structured when bound to its target, sigma 28. Nat Struct Biol 4 285–291 [DOI] [PubMed] [Google Scholar]
- Dawson R, Muller L, Dehner A, Klein C, Kessler H, Buchner J (2003) The N-terminal domain of p53 is natively unfolded. J Mol Biol 332 1131–1141 [DOI] [PubMed] [Google Scholar]
- Dedmon MM, Patel CN, Young GB, Pielak GJ (2002) FlgM gains structure in living cells. Proc Natl Acad Sci USA 99 12681–12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z (2002) Intrinsic disorder and protein function. Biochemistry 41 6573–6582 [DOI] [PubMed] [Google Scholar]
- Ellis RJ (2001. a) Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol 11 114–119 [DOI] [PubMed] [Google Scholar]
- Ellis RJ (2001. b) Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci 26 597–604 [DOI] [PubMed] [Google Scholar]
- Fink AL (2005) Natively unfolded proteins. Curr Opin Struct Biol 15 35–41 [DOI] [PubMed] [Google Scholar]
- Fink AL (2006) The aggregation and fibrillation of alpha-synuclein. Acc Chem Res 39 628–634 [DOI] [PubMed] [Google Scholar]
- Flaugh SL, Lumb KJ (2001) Effects of macromolecular crowding on the intrinsically disordered proteins c-Fos and p27(Kip1). Biomacromolecules 2 538–540 [DOI] [PubMed] [Google Scholar]
- Foord RL, Leatherbarrow RJ (1998) Effect of osmolytes on the exchange rates of backbone amide protons in proteins. Biochemistry 37 2969–2978 [DOI] [PubMed] [Google Scholar]
- Garay-Arroyo A, Colmenero-Flores JM, Garciarrubio A, Covarrubias AA (2000) Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J Biol Chem 275 5668–5674 [DOI] [PubMed] [Google Scholar]
- Goers J, Uversky VN, Fink AL (2003) Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci 12 702–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal K, Pinelli C, Maslen SL, Rastogi RK, Stephens E, Tunnacliffe A (2005) Dehydration-regulated processing of late embryogenesis abundant protein in a desiccation-tolerant nematode. FEBS Lett 579 4093–4098 [DOI] [PubMed] [Google Scholar]
- Goyal K, Tisi L, Basran A, Browne J, Burnell A, Zurdo J, Tunnacliffe A (2003) Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J Biol Chem 278 12977–84 [DOI] [PubMed] [Google Scholar]
- Hall D (2006) Protein self-association in the cell: a mechanism for fine tuning the level of macromolecular crowding? Eur Biophys J 35 276–280 [DOI] [PubMed] [Google Scholar]
- Hall D, Dobson CM (2006) Expanding to fill the gap: a possible role for inert biopolymers in regulating the extent of the ‘macromolecular crowding’ effect. FEBS Lett 580 2584–2590 [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51 463–499 [DOI] [PubMed] [Google Scholar]
- Hedberg L, Oliveberg M (2004) Scattered Hammond plots reveal second level of site-specific information in protein folding: phi′ (beta++). Proc Natl Acad Sci USA 101 7606–7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J (2001. a) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6 431–438 [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Tetteroo FA, Wolkers WF (2001. b) Induction of desiccation tolerance in plant somatic embryos: how exclusive is the protective role of sugars? Cryobiology 43 140–150 [DOI] [PubMed] [Google Scholar]
- Holthauzen LM, Bolen DW (2007) Mixed osmolytes: the degree to which one osmolyte affects the protein stabilizing ability of another. Protein Sci 16 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47 377–403 [DOI] [PubMed] [Google Scholar]
- Ittah V, Kahana E, Amir D, Haas E (2004) Applications of time-resolved resonance energy transfer measurements in studies of the molecular crowding effect. J Mol Recognit 17 448–455 [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang Y (2004) Beta-elimination coupled with tandem mass spectrometry for the identification of in vivo and in vitro phosphorylation sites in maize dehydrin DHN1 protein. Biochemistry 43 15567–15576 [DOI] [PubMed] [Google Scholar]
- Klimov DK, Newfield D, Thirumalai D (2002) Simulations of beta-hairpin folding confined to spherical pores using distributed computing. Proc Natl Acad Sci USA 99 8019–8024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koag MC, Fenton RD, Wilkens S, Close TJ (2003) The binding of maize DHN1 to lipid vesicles: gain of structure and lipid specificity. Plant Physiol 131 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblatt JA, Kornblatt MJ (2002) The effects of osmotic and hydrostatic pressures on macromolecular systems. Biochim Biophys Acta 1595 30–47 [DOI] [PubMed] [Google Scholar]
- Kovacs D, Kalmar E, Torok Z, Tompa P (2008) Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol 147 381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozer N, Schreiber G (2004) Effect of crowding on protein-protein association rates: fundamental differences between low and high mass crowding agents. J Mol Biol 336 763–774 [DOI] [PubMed] [Google Scholar]
- Lattman EE, Fiebig KM, Dill KA (1994) Modeling compact denatured states of proteins. Biochemistry 33 6158–6166 [DOI] [PubMed] [Google Scholar]
- Lisse T, Bartels D, Kalbitzer HR, Jaenicke R (1996) The recombinant dehydrin-like desiccation stress protein from the resurrection plant Craterostigma plantagineum displays no defined three-dimensional structure in its native state. Biol Chem 377 555–561 [DOI] [PubMed] [Google Scholar]
- Love JJ, Li X, Chung J, Dyson HJ, Wright PE (2004) The LEF-1 high-mobility group domain undergoes a disorder-to-order transition upon formation of a complex with cognate DNA. Biochemistry 43 8725–8734 [DOI] [PubMed] [Google Scholar]
- Luo P, Baldwin RL (1997) Mechanism of helix induction by trifluoroethanol: a framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 36 8413–8421 [DOI] [PubMed] [Google Scholar]
- McNulty BC, Tripathy A, Young GB, Charlton LM, Orans J, Pielak GJ (2006. a) Temperature-induced reversible conformational change in the first 100 residues of alpha-synuclein. Protein Sci 15 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty BC, Young GB, Pielak GJ (2006. b) Macromolecular crowding in the Escherichia coli periplasm maintains alpha-synuclein disorder. J Mol Biol 355 893–897 [DOI] [PubMed] [Google Scholar]
- Minton AP (2005. a) Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J Pharm Sci 94 1668–1675 [DOI] [PubMed] [Google Scholar]
- Minton AP (2005. b) Models for excluded volume interaction between an unfolded protein and rigid macromolecular cosolutes: macromolecular crowding and protein stability revisited. Biophys J 88 971–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton AP (2006) Macromolecular crowding. Curr Biol 16 R269–271 [DOI] [PubMed] [Google Scholar]
- Morar AS, Olteanu A, Young GB, Pielak GJ (2001) Solvent-induced collapse of alpha-synuclein and acid-denatured cytochrome c. Protein Sci 10 2195–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillon JM, Gustafsson P, Harryson P (2006) Structural investigation of disordered stress proteins: comparison of full-length dehydrins with isolated peptides of their conserved segments. Plant Physiol 141 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveberg M, Wolynes PG (2005) The experimental survey of protein-folding energy landscapes. Q Rev Biophys 38 245–288 [DOI] [PubMed] [Google Scholar]
- Otzen DE, Oliveberg M (1999) Salt-induced detour through compact regions of the protein folding landscape. Proc Natl Acad Sci USA 96 11746–11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otzen DE, Oliveberg M (2002) Conformational plasticity in folding of the split beta-alpha-beta protein S6: evidence for burst-phase disruption of the native state. J Mol Biol 317 613–627 [DOI] [PubMed] [Google Scholar]
- Qu Y, Bolen CL, Bolen DW (1998) Osmolyte-driven contraction of a random coil protein. Proc Natl Acad Sci USA 95 9268–9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Bolen DW (2002) Efficacy of macromolecular crowding in forcing proteins to fold. Biophys Chem 101–102 155–165 [DOI] [PubMed] [Google Scholar]
- Ren X, Yang Z, Kuang T (2006) Solvent-induced changes in photochemical activity and conformation of photosystem I particles by glycerol. Biol Chem 387 23–29 [DOI] [PubMed] [Google Scholar]
- Roberts JK, DeSimone NA, Lingle WL, Dure L III (1993) Cellular concentrations and uniformity of cell-type accumulation of two lea proteins in cotton embryos. Plant Cell 5 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrig H, Schmidt J, Colby T, Brautigam A, Hufnagel P, Bartels D (2006) Desiccation of the resurrection plant Craterostigma plantagineum induces dynamic changes in protein phosphorylation. Plant Cell Environ 29 1606–1617 [DOI] [PubMed] [Google Scholar]
- Sasakawa H, Sakata E, Yamaguchi Y, Masuda M, Mori T, Kurimoto E, Iguchi T, Hisanaga S, Iwatsubo T, Hasegawa M, et al (2007) Ultra-high field NMR studies of antibody binding and site-specific phosphorylation of alpha-synuclein. Biochem Biophys Res Commun 363 795–799 [DOI] [PubMed] [Google Scholar]
- Schellman JA (2002) Fifty years of solvent denaturation. Biophys Chem 96 91–101 [DOI] [PubMed] [Google Scholar]
- Selenko P, Serber Z, Gadea B, Ruderman J, Wagner G (2006) Quantitative NMR analysis of the protein G B1 domain in Xenopus laevis egg extracts and intact oocytes. Proc Natl Acad Sci USA 103 11904–11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silow M, Oliveberg M (2003) High concentrations of viscogens decrease the protein folding rate constant by prematurely collapsing the coil. J Mol Biol 326 263–271 [DOI] [PubMed] [Google Scholar]
- Soulages JL, Kim K, Arrese EL, Walters C, Cushman JC (2003) Conformation of a group 2 late embryogenesis abundant protein from soybean: evidence of poly (L-proline)-type II structure. Plant Physiol 131 963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulages JL, Kim K, Walters C, Cushman JC (2002) Temperature-induced extended helix/random coil transitions in a group 1 late embryogenesis-abundant protein from soybean. Plant Physiol 128 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street TO, Bolen DW, Rose GD (2006) A molecular mechanism for osmolyte-induced protein stability. Proc Natl Acad Sci USA 103 13997–14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Palva ET, Welin B (2000) Purification of recombinant Arabidopsis thaliana dehydrins by metal ion affinity chromatography. Protein Expr Purif 20 169–178 [DOI] [PubMed] [Google Scholar]
- Tolleter D, Jaquinod M, Mangavel C, Passirani C, Saulnier P, Manon S, Teyssier E, Payet N, Avelange-Macherel MH, Macherel D (2007) Structure and function of a mitochondrial late embryogenesis abundant protein are revealed by desiccation. Plant Cell 19 1580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa P, Csermely P (2004) The role of structural disorder in the function of RNA and protein chaperones. FASEB J 18 1169–1175 [DOI] [PubMed] [Google Scholar]
- Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439 688–694 [DOI] [PubMed] [Google Scholar]
- Uversky VN, Oldfield CJ, Dunker AK (2005) Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit 18 343–384 [DOI] [PubMed] [Google Scholar]
- Venkatesu P, Lee MJ, Lin HM (2007) Thermodynamic characterization of the osmolyte effect on protein stability and the effect of GdnHCl on the protein denatured state. J Phys Chem B 111 9045–9056 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218 1–14 [DOI] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA (2004) DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res 32 W668–W673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA (2001) Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta 1544 196–206 [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38 1095–1102 [DOI] [PubMed] [Google Scholar]
- Zimmerman SB, Minton AP (1993) Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu Rev Biophys Biomol Struct 22 27–65 [DOI] [PubMed] [Google Scholar]
- Zimmerman SB, Trach SO (1991) Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol 222 599–620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.