Abstract
Hematopoietic stem cell transplantation (HSCT) is often the only practical approach to fatal genetic defects. One of the first pathologies which HSCT was applied to was Autosomal Recessive Osteopetrosis (ARO), a rare genetic bone disease in which a deficit in bone resorption by osteoclasts leads to increased bone density and secondary defects. The disease is often lethal early in life unless treated with HSCT. In utero transplantation (IUT) of the oc/oc mouse, reproducing the clinical features of a subset of ARO, has demonstrated that the quality of life and the survival of transplanted animals are greatly improved, suggesting that a similar protocol could be applied to humans. However, recently the dissection of the molecular bases of the disease has shown that ARO is genetically heterogeneous and has revealed the presence of subsets of patients which do not benefit from HSCT. This observation highlights the importance of molecular diagnosing ARO to identify and establish the proper therapies for a better prognosis. In particular, on the basis of experimental results in murine models, efforts should be undertaken to develop approaches such as IUT and new pharmacological strategies.
Keywords: Osteoclast, Transplantation, Stem cells, Differentiation
Introduction
Bone is a dynamic tissue in which osteoblasts synthesize bone matrix and osteoclasts resorb bone, in order to assure normal skeletal development (growth and remodelling), and the maintenance of its integrity throughout life. Therefore, bone density is dependent on the balance between the activities of these two cell types, which display their antagonistic functions under the regulation of osteotropic and calciotropic hormones (Hadjidakis and Androulakis 2006). The disruption of this balance in pathologic conditions leads to phenotypes characterised by either a reduction or an increase in bone mass. In this regard, the osteopetroses are a group of inherited bone diseases in which failure in osteoclast resorptive activity causes a generalized increase in bone mass. Human osteopetrosis is clinically and genetically heterogeneous and at least four different forms can be distinguished on the basis of severity and age of onset. In particular, Autosomal Recessive Osteopetrosis (ARO), also called infantile malignant, presents early in life with extreme sclerosis of the skeleton, reduction of bone marrow spaces leading to anemia and hepatosplenomegaly due to secondary hematopoiesis, cranial nerves compression and severe growth failure. The disease is often lethal within the first decade of life because of secondary infections. Two forms can be distinguished on the basis of the presence or absence of osteoclasts in bone, as demonstrated by bone biopsy: the osteoclast-rich form, in which osteoclasts are present in a normal to high number, but cannot resorb bone; and the recently identified osteoclast-poor form, in which osteoclasts are absent because of a defect in the osteoclastogenic process.
Five genes have been involved in the pathogenesis of human ARO, so far: TCIRG1 (Frattini et al. 2000), ClCN7 (Kornak et al. 2001), OSTM1 (Chalhoub et al. 2003), PLEKHM1 (Van Wesenbeeck et al. 2007), leading to an osteoclast-rich phenotype; and RANKL (Sobacchi et al. 2007), the only gene recognised to date as responsible for the osteoclast-poor phenotype. Moreover it is likely that the involvement of other genes still has to be demonstrated, as suggested by the fact that in a good percentage of cases no mutations in the known genes have been found.
So far, the only available treatment for ARO is hematopoietic stem cells transplantation (HSCT), an approach performed since the early 1980s (Coccia et al. 1980) and based on the fact that osteoclasts derive from precursors of the monocytic/macrophage lineage. However, the better understanding of the genetic defects leading to ARO has highlighted the need for new therapeutic approaches to this disease, including both cellular and pharmacological strategies.
In the present work, we will comment on the experience of our group, which has collected a large cohort of about 230 ARO patients in the last years, classified on the basis of the specific genetic defect. We will highlight the correlations between genotype and outcome of HSCT; finally we will address the issue of developing new therapies in order to get to a better prognosis of the disease.
Treating human ARO: the osteoclast-rich forms
In 1980 Coccia and colleagues reported on the first bone marrow transplantation on a five-month-old girl affected with ARO, which received the transplant from her HLA-identical elder brother after preparation with cyclophosphamide and total body irradiation (Coccia et al. 1980). After the transplant, engraftment was documented by chromosomal analysis; the evaluation of several parameters, such as recovery of the haematological defect, normalization of serum levels of calcium and alkaline phosphatase, X-ray imaging of the bone tissue and evaluation of bone biopsy specimens, allowed the authors to determine the presence of new, actively resorbing osteoclasts, of donor origin, in the bone of the treated girl, and to conclude that allogeneic bone marrow transplantation could be considered the treatment of choice for this fatal disease. This has been widely accepted for more then 20 years and confirmed by a series of papers evaluating the experience, in this field, of single centers (Gerritsen et al. 1994; Schulz et al. 2002; Tsuji et al. 2005; Corbacioglu et al. 2006; Jaing et al. 2006; Tolar et al. 2006; and others). The largest study published so far is by Driessen and colleagues, which performed a retrospective analysis of 122 children who had received an HSCT between 1980 and 2001 (Driessen et al. 2003). The data collected showed that in general HSCT had to be considered the first choice treatment for ARO, the best results being obtained with patients receiving a genotypic HLA-matched HSCT and thus gaining survival probabilities much higher than patients receiving other kinds of graft (73% versus a probability not higher than 43%, in the case of phenotypic HLA-identical or one HLA-antigen mismatched related donor, or even lower). A second conclusion of the authors was that the best results, in terms of rescue of secondary defects such as the visual impairment, could be obtained when the age of the transplanted patient was younger.
However, in the last 8 years the understanding of the molecular bases of ARO has greatly improved: 5 genes have been identified as responsible for the disease (TCIRG1, ClCN7, OSTM1, PLEKHM1, RANKL) and important genotype-phenotype correlations have been established, while the search for new genes involved in its pathogenesis continues in the subgroup of patients without known genetic defect.
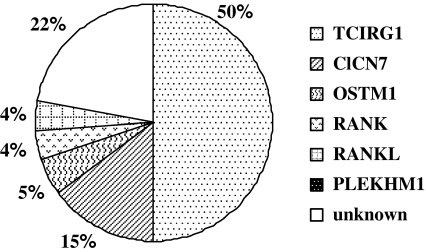
In our cohort of 230 individuals affected by ARO, careful analysis of genetic and clinical data has allowed us to clearly show the link between the specific gene affected in a given patient and the outcome of classical therapies (namely, HSCT). In the 50% of cases (Fig. 1), the disease is due to mutations in TCIRG1 (Frattini et al. 2000; Kornak et al. 2000; Sobacchi et al. 2001; Michigami et al. 2002; Scimeca et al. 2003; Susani et al. 2004; Souraty et al. 2007) which encodes for the osteoclast-specific a3 subunit of the proton pump responsible for the acidification of the resorption lacuna leading to bone resorption. Patients bearing mutations in this gene display a classical, homogeneous osteopetrotic phenotype and respond well to HSCT, when a suitable donor is available.
Fig. 1.
Distribution, among the known genes, of the mutations found in our cohort of 230 ARO patients
Patients with defects in ClCN7 gene, which encodes for a chloride channel essential for osteoclast function, represent the 15% of cases and are characterized by a spectrum of phenotypes ranging from severe to mild form (Kornak et al. 2001; Cleiren et al. 2001; Frattini et al. 2003; Campos-Xavier et al. 2005). The severe forms also present with neurological symptoms, such as retinal degeneration and cerebral atrophy, suggested to be primary and not secondary to the bone defect, as it occurs in the TCIRG1-dependent forms. The opportunity of HSCT in these patients has to be carefully evaluated and exhaustive CNS examination is suggested, considering that, even in case of engraftment, the neurological deficits are not rescued.
The subset of patients (5% in our cohort) displaying mutations in OSTM1 gene, encoding for a protein involved in late endosomal-lysosomal trafficking, functionally and physically linked to ClCN7 (Lange et al. 2006; Meadows et al. 2007), is characterized by even more serious neurological defects (neurodegeneration, optic atrophy, microcephaly, cortical atrophy; in one case, bilateral atrial subependymal heterotopias) (Chalhoub et al. 2003; Quarello et al. 2004; Ramirez et al. 2004; Pangrazio et al. 2006; Castellano Chiodo et al. 2007; Maranda et al. 2008). These findings strongly discourage HSCT, because, even if the transplant engrafted, the patient would die due to the severe CNS defects; indeed, in our series no OSTM1-dependent patients have been transplanted.
Regarding PLEKHM1, whose gene product has been suggested to take part into osteoclastic vesicular traffic, only one case has been reported, displaying an intermediate phenotype, not requiring HSCT (Van Wesenbeeck et al. 2007).
Treating human ARO: the osteoclast-poor form
Recently a peculiar subset of human ARO has been identified as characterized by the absence of osteoclasts in the bone biopsy specimens of the patients (Flanagan et al. 2002; Nicholls et al. 2005); therefore it has been named osteoclast-poor, in contrast with the osteoclast-rich, in which a normal to high number of nonfunctional osteoclasts is present. In the osteoclast-poor subset a defect in the osteoclastogenic process has been suggested and indeed last year our group reported the identification of mutations in RANKL gene in 6 ARO patients (Sobacchi et al. 2007) and, more recently, in two more patients (unpublished data). RANKL protein, expressed on the plasma membrane of stromal cells and osteoblasts, has been demonstrated, both in vivo and in vitro, to be essential for osteoclast differentiation from their precursors (Lacey et al. 1998; Kong et al. 1999; Kim et al. 2000); in fact, when tested in an in vitro differentiation assay, in the presence of the wild-type, recombinant cytokine, PBMCs from RANKL-dependent patients can properly differentiate into mature, functional osteoclasts (Sobacchi et al. 2007). Interestingly 4 out of 8 RANKL-dependent patients failed to rescue the bone defect after HSCT engraftment; however, this result is not unexpected, since in this subset of ARO the affected protein comes from cells (the osteoblasts) of mesenchymal, and not haematopoietic origin. Therefore, in this case the specific genetic defect makes HSCT an approach completely inappropriate to cure the disease and highlights the need for new therapeutical approaches.
Very recently our group could identify another subset of osteoclast-poor ARO associated with hypogammaglobulinemia, bearing mutations not in RANKL gene, but in the gene encoding its cognate receptor RANK (Guerrini et al. 2008). This finding is important because it confirms that also osteoclast-poor ARO is genetically heterogeneous, and it has therapeutical implications. In fact, in our group of 8 RANK-dependent patients, out of 4 which received HSCT, 2 could rescue the bone defect, while the remaining died due to complications related to the therapeutic intervention itself; these data show that, as expected, this new subset of human ARO can be successfully treated with HSCT, resembling TCIRG1-dependent individuals.
Conclusions and perspectives
Recent advances in the molecular diagnosis of human ARO have clearly shown its relevance with respect to clinical management of the patients and prognosis of the disease. They have suggested that HSCT should be offered to patients only after a precise genetic investigation, being the possibilities to rescue the disease strictly related to the specific genetic defect found in each subject. However, in the subgroup of patients which could potentially benefit from HSCT, this should be performed as soon as possible, in order to avoid the establishment of irreversible secondary defects, such as blindness and deafness. Studies in a murine model of osteopetrosis, the oc/oc mouse, bearing a defect in tcirg1 gene, demonstrated that in utero transplantation of HSC can rescue the phenotype in the treated mice (Frattini et al. 2005) and it has also the advantage that recipients don’t need to be ablated and immunosuppressed; moreover, in all pups born after in utero treatment, graft-versus-host disease was not observed, which, when it occurs in man, is often a cause of death. Therefore the same approach should be taken into consideration also in man, when a prenatal diagnosis of osteopetrosis can be drawn, in order to gain the maximum benefits from HSCT, as it is already done in some genetic, immunological diseases (Weinberg et al. 2001).
On a clinical point of view, the identification of the subset of RANKL-dependent ARO patients represents a challenge: while these affected individuals do not respond to HSCT, they could take advantage from mesenchymal stem cell transplantation (MSCT). The potentialities of this kind of approach are currently under consideration in several fields, as demonstrated by the growing list of publications on this topic (Corsten and Shah 2008; Granero-Molto et al. 2008; Liu et al. 2008; Meyerrose et al. 2008; Xu and Liu 2008; and others). The rationale in the application of MSCT to RANKL-dependent ARO is that in this way the patient would receive the specific precursor cells able to differentiate into normal osteoblasts, producing in situ the cytokine RANKL, which is defective in the recipient, and thus allowing the differentiation of osteoclast precursors into normal, functional cells. Besides the possibility of exploiting cellular approaches other than HSCT, the identification of RANKL-dependent ARO paves the way also to pharmacological approaches, as, at least in theory, these patients could benefit from the administration of recombinant RANKL. For this purpose studies in animal models are required and, if they produce positive results supporting the feasibility of this approach also in man, a major effort will be needed in order to make biotechnological firms meet the world of “orphan” diseases.
Acknowledgements
This work was supported by grants from Eurostells (STELLAR) and FIRB/MIUR to P.V. (RBIN04CHXT), from the Fondazione Telethon to C.S. (grant GGP07059), from the Fondazione Cariplo to A.F and from ISS Malattie Rare (New cell therapy approaches for infantile malignant Osteopetrosis) to P.V. The work reported in this paper has also been funded by the N.O.B.E.L. (Network Operativo per la Biomedicina di Eccellenza in Lombardia) Program from Fondazione Cariplo to P.V. and A.V. The technical assistance of Dario Strina and Lucia Susani is acknowledged.
References
- Campos-Xavier AB, Casanova JL, Doumaz Y, Feingold J, Munnich A, Cormier-Daire V (2005) Intrafamilial phenotypic variability of osteopetrosis due to chloride channel 7 (CLCN7) mutations. Am J Med Genet A 133A:216–218. doi:10.1002/ajmg.a.30490 [DOI] [PubMed]
- Castellano Chiodo D, DiRocco M, Gandolfo C, Morana G, Buzzi D, Rossi A (2007) Neuroimaging findings in malignant infantile osteopetrosis due to OSTM1 mutations. Neuropediatrics 38:154–156. doi:10.1055/s-2007-990267 [DOI] [PubMed]
- Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A, Villa A, Vacher J (2003) Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med 9:399–406. doi:10.1038/nm842 [DOI] [PubMed]
- Cleiren E, Bénichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, de Vernejoul MC, Van Hul W (2001) Albers-Schönberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 10:2861–2867. doi:10.1093/hmg/10.25.2861 [DOI] [PubMed]
- Coccia PF, Krivit W, Cervenka J, Clawson C, Kersey JH, Kim TH, Nesbit ME, Ramsay NK, Warkentin PI, Teitelbaum SL, Kahn AJ, Brown DM (1980) Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med 302:701–708 [DOI] [PubMed]
- Corbacioglu S, Hönig M, Lahr G, Stöhr S, Berry G, Friedrich W, Schulz AS (2006) Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant 38:547–553. doi:10.1038/sj.bmt.1705485 [DOI] [PubMed]
- Corsten MF, Shah K (2008) Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol 9:376–384R. doi:10.1016/S1470-2045(08)70099-8 [DOI] [PubMed]
- Driessen GJ, Gerritsen EJ, Fischer A, Fasth A, Hop WC, Veys P, Porta F, Cant A, Steward CG, Vossen JM, Uckan D, Friedrich W (2003) Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant 32:657–663. doi:10.1038/sj.bmt.1704194 [DOI] [PubMed]
- Flanagan AM, Massey HM, Wilson C, Vellodi A, Horton MA, Steward CG (2002) Macrophage colony-stimulating factor and receptor activator NF-kappaB ligand fail to rescue osteoclast-poor human malignant infantile osteopetrosis in vitro. Bone 30:85–90. doi:10.1016/S8756-3282(01)00656-1 [DOI] [PubMed]
- Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A (2000) Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25:343–346. doi:10.1038/77131 [DOI] [PubMed]
- Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M, Abinun M, Andolina M, Flanagan A, Horwitz EM, Mihci E, Notarangelo LD, Ramenghi U, Teti A, Van Hove J, Vujic D, Young T, Albertini A, Orchard PJ, Vezzoni P, Villa A (2003) Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res 18:1740–1747. doi:10.1359/jbmr.2003.18.10.1740 [DOI] [PubMed]
- Frattini A, Blair HC, Sacco MG, Cerisoli F, Faggioli F, Catò EM, Pangrazio A, Musio A, Rucci F, Sobacchi C, Sharrow AC, Kalla SE, Bruzzone MG, Colombo R, Magli MC, Vezzoni P, Villa A (2005) Rescue of ATPa3-deficient murine malignant osteopetrosis by hematopoietic stem cell transplantation in utero. Proc Natl Acad Sci USA 102:14629–14634. doi:10.1073/pnas.0507637102 [DOI] [PMC free article] [PubMed]
- Gerritsen EJ, Vossen JM, Fasth A, Friedrich W, Morgan G, Padmos A, Vellodi A, Porras O, O’Meara A, Porta F et al (1994) Bone marrow transplantation for autosomal recessive osteopetrosis. A report from the Working Party on Inborn Errors of the European Bone Marrow Transplantation Group. J Pediatr 125:896–902. doi:10.1016/S0022-3476(05)82004-9 [DOI] [PubMed]
- Granero-Molto F, Weis JA, Longobardi L, Spagnoli A (2008) Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 8:255–268. doi:10.1517/14712598.8.3.255 [DOI] [PubMed]
- Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A, Moratto D, Mazzolari E, Clayton-Smith J, Orchard P, Coxon FP, Helfrich MH, Crockett JC, Mellis D, Vellodi A, Tezcan I, Notarangelo LD, Rogers MJ, Vezzoni P, Villa A, Frattini A (2008) Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 83:64–76 [DOI] [PMC free article] [PubMed]
- Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann NY Acad Sci 1092:385–396. doi:10.1196/annals.1365.035 [DOI] [PubMed]
- Jaing TH, Hou JW, Chen SH, Huang IA, Wang CJ, Lee WI (2006) Successful unrelated mismatched cord blood transplantation in a child with malignant infantile osteopetrosis. Pediatr Transplant 10:629–631. doi:10.1111/j.1399-3046.2006.00537.x [DOI] [PubMed]
- Kim N, Odgren PR, Kim DK, Marks SC Jr, Choi Y (2000) Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA 97:10905–10910. doi:10.1073/pnas.200294797 [DOI] [PMC free article] [PubMed]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323. doi:10.1038/16852 [DOI] [PubMed]
- Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C (2000) Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 9:2059–2063. doi:10.1093/hmg/9.13.2059 [DOI] [PubMed]
- Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215. doi:10.1016/S0092-8674(01)00206-9 [DOI] [PubMed]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shaloub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176. doi:10.1016/S0092-8674(00)81569-X [DOI] [PubMed]
- Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC (2006) ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 440:220–223. doi:10.1038/nature04535 [DOI] [PubMed]
- Liu G, Shu C, Cui L, Liu W, Cao Y (2008) Tissue-engineered bone formation with cryopreserved human bone marrow mesenchymal stem cells. Cryobiology 56:209–215. doi:10.1016/j.cryobiol.2008.02.008 [DOI] [PubMed]
- Maranda B, Chabot G, Décarie JC, Pata M, Azeddine B, Moreau A, Vacher J (2008) Clinical and cellular manifestations of OSTM1-related infantile osteopetrosis. J Bone Miner Res 23:296–300. doi:10.1359/jbmr.071015 [DOI] [PubMed]
- Meadows NA, Sharma SM, Faulkner GJ, Ostrowski MC, Hume DA, Cassady AI (2007) The expression of chloride channel 7 (ClCN7) and OSTM1 in osteoclasts is co-regulated by microphtalmia transcription factor. J Biol Chem 282:1891–1904. doi:10.1074/jbc.M608572200 [DOI] [PubMed]
- Meyerrose TE, Roberts M, Ohlemiller KK, Vogler CA, Wirthlin L, Nolta JA, Sands MS (2008) Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells 26:1713–1722 [DOI] [PMC free article] [PubMed]
- Michigami T, Kageyama T, Satomura K, Shima M, Yamaoka K, Nakayama M, Ozono K (2002) Novel mutations in the a3 subunit of vacuolar H(+)-adenosine triphosphatase in a Japanese patient with infantile malignant osteopetrosis. Bone 30:436–439. doi:10.1016/S8756-3282(01)00684-6 [DOI] [PubMed]
- Nicholls BM, Bredius RG, Hamdy NA, Gerritsen EJ, Lankester AC, Hogendoorn PC, Nesbitt SA, Horton MA, Flanagan AM (2005) Limited rescue of osteoclast-poor osteopetrosis after successful engraftment by cord blood from an unrelated donor. J Bone Miner Res 20:2264–2270. doi:10.1359/JBMR.050807 [DOI] [PubMed]
- Pangrazio A, Poliani PL, Megarbane A, Lefranc G, Lanino E, Di Rocco M, Rucci F, Lucchini F, Ravanini M, Facchetti F, Abinum M, Vezzoni P, Villa A, Frattini A (2006) Mutations in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J Bone Miner Res 21:1098–1105. doi:10.1359/jbmr.060403 [DOI] [PubMed]
- Quarello P, Forni M, Barberis L, Defilippi C, Campagnoli MF, Silvestro L, Frattini A, Chalhoub N, Vacher J, Ramenghi U (2004) Severe malignant osteopetrosis caused by a GL gene mutation. J Bone Miner Res 19:1194–1199. doi:10.1359/JBMR.040407 [DOI] [PubMed]
- Ramirez A, Faupel J, Goebel I, Stiller A, Beyer S, Stockle C, Hasan C, Bode U, Kornak U, Kubisch C (2004) Identification of a novel mutation in the coding region of the grey-lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum Mutat 23:471–476. doi:10.1002/humu.20028 [DOI] [PubMed]
- Schulz AS, Classen CF, Mihatsch WA, Sigl-Kraetzig M, Wiesneth M, Debatin KM, Friedrich W, Müller SM (2002) HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood 99:3458–3460. doi:10.1182/blood.V99.9.3458 [DOI] [PubMed]
- Scimeca JC, Quincey D, Parrinello H, Romatet D, Grosgeorge J, Gaudray P, Philip N, Fischer A, Carle GF (2003) Novel mutations in the TCIRG1 gene encoding the a3 subunit of the vacuolar proton pump in patients affected by infantile malignant osteopetrosis. Hum Mutat 21:151–157. doi:10.1002/humu.10165 [DOI] [PubMed]
- Sobacchi C, Frattini A, Orchard P, Porras O, Tezcan I, Andolina M, Babul-Hirji R, Baric I, Canham N, Chitayat D, Dupuis-Girod S, Ellis I, Etzioni A, Fasth A, Fisher A, Gerritsen B, Gulino V, Horwitz E, Klamroth V, Lanino E, Mirolo M, Musio A, Matthijs G, Nonomaya S, Notarangelo LD, Ochs HD, Superti Furga A, Valiaho J, van Hove JL, Vihinen M, Vujic D, Vezzoni P, Villa A (2001) The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum Mol Genet 10:1767–1773. doi:10.1093/hmg/10.17.1767 [DOI] [PubMed]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, Grabowski P, Del Fattore A, Messina C, Errigo G, Coxon FP, Scott DI, Teti A, Rogers MJ, Vezzoni P, Villa A, Helfrich MH (2007) Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39:960–962. doi:10.1038/ng2076 [DOI] [PubMed]
- Souraty N, Noun P, Djambas-Khayat C, Chouery E, Pangrazio A, Villa A, Lefranc G, Frattini A, Mégarbané A (2007) Molecular study of six families originating from the Middle-East and presenting with autosomal recessive osteopetrosis. Eur J Med Genet 50:188–199. doi:10.1016/j.ejmg.2007.01.005 [DOI] [PubMed]
- Susani L, Pangrazio A, Sobacchi C, Taranta A, Mortier G, Savarirayan R, Villa A, Orchard P, Vezzoni P, Albertini A, Frattini A, Pagani F (2004) TCIRG1-dependent recessive osteopetrosis: mutation analysis, functional identification of the splicing defects and in vitro rescue by U1snRNA. Hum Mutat 24:225–235. doi:10.1002/humu.20076 [DOI] [PubMed]
- Tolar J, Bonfim C, Grewal S, Orchard P (2006) Engraftment and survival following hematopoietic stem cell transplantation for osteopetrosis using a reduced intensity conditioning regimen. Bone Marrow Transplant 38:783–787. doi:10.1038/sj.bmt.1705533 [DOI] [PubMed]
- Tsuji Y, Ito S, Isoda T, Kajiwara M, Nagasawa M, Morio T, Mizutani S (2005) Successful nonmyeloablative cord blood transplantation for an infant with malignant infantile osteopetrosis. J Pediatr Hematol Oncol 27:495–498. doi:10.1097/01.mph.0000179961.72889.bf [DOI] [PubMed]
- Van Wesenbeeck L, Odgren PR, Coxon FP, Frattini A, Moens P, Perdu B, MacKay CA, Van Hul E, Timmermans JP, Vanhoenacker F, Jacobs R, Peruzzi B, Teti A, Helfrich MH, Rogers MJ, Villa A, Van Hul W (2007) Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest 117:919–930. doi:10.1172/JCI30328 [DOI] [PMC free article] [PubMed]
- Weinberg KI, Kapoor N, Shah AJ, Crooks GM, Kohn DB, Parkman R (2001) Hematopoietic stem cell transplantation for severe combined immune deficiency. Curr Allergy Asthma Rep 1:416–420R. doi:10.1007/s11882-001-0026-2 [DOI] [PubMed]
- Xu YQ, Liu ZC (2008) Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev 4:101–112 [DOI] [PubMed]