Abstract
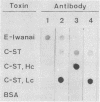
The partial amino acid sequence of the light-chain (Lc) component of Clostridium botulinum type C1 toxin was determined. The sequence was quite similar to those of the other types of botulinum and tetanus toxins. Nine monoclonal antibodies against botulinum type E toxin were established by immunizing BALB/c mice with type E toxoid or its Lc component. Six antibodies reacted with the heavy-chain component and three reacted with the Lc component of the toxin. One of the latter three antibodies reacted with botulinum type B, C1, and D toxins and tetanus toxin, as well as botulinum type E toxin. This antibody recognized the Lc components of these toxins, indicating that there exists one common antigenic determinant on the Lc regions of these toxins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eisel U., Jarausch W., Goretzki K., Henschen A., Engels J., Weller U., Hudel M., Habermann E., Niemann H. Tetanus toxin: primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986 Oct;5(10):2495–2502. doi: 10.1002/j.1460-2075.1986.tb04527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Kamata Y., Nagai T., Ogasawara J., Sakaguchi G. The use of monoclonal antibodies to analyze the structure of Clostridium botulinum type E derivative toxin. Infect Immun. 1986 Jun;52(3):786–791. doi: 10.1128/iai.52.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishi S., Yamazaki H. Monoclonal antibodies against AFP: application in immunoassay and affinity chromatography. Ann N Y Acad Sci. 1983;417:452–458. doi: 10.1111/j.1749-6632.1983.tb32887.x. [DOI] [PubMed] [Google Scholar]
- Oguma K., Agui T., Syuto B., Kimura K., Iida H., Kubo S. Four different monoclonal antibodies against type C1 toxin of Clostridium botulinum. Infect Immun. 1982 Oct;38(1):14–20. doi: 10.1128/iai.38.1.14-20.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Murayama S., Syuto B., Iida H., Kubo S. Analysis of antigenicity of Clostridium botulinum type C1 and D toxins by polyclonal and monoclonal antibodies. Infect Immun. 1984 Feb;43(2):584–588. doi: 10.1128/iai.43.2.584-588.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Syuto B., Iida H., Kubo S. Antigenic similarity of toxins produced by Clostridium botulinum type C and D strains. Infect Immun. 1980 Dec;30(3):656–660. doi: 10.1128/iai.30.3.656-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. Oral toxicities of Clostridium botulinum type A and B toxins from different strains. Infect Immun. 1984 Feb;43(2):487–490. doi: 10.1128/iai.43.2.487-490.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I., Sakaguchi G. Purification of Clostridium botuliunum type F progenitor toxin. Appl Microbiol. 1974 Dec;28(6):923–928. doi: 10.1128/am.28.6.923-928.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyamoorthy V., DasGupta B. R. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J Biol Chem. 1985 Sep 5;260(19):10461–10466. [PubMed] [Google Scholar]
- Syuto B., Kubo S. Isolation and molecular size of Clostridium botulinum type C toxin. Appl Environ Microbiol. 1977 Feb;33(2):400–405. doi: 10.1128/aem.33.2.400-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Separation and characterization of heavy and light chains from Clostridium botulinum type C toxin and their reconstitution. J Biol Chem. 1981 Apr 25;256(8):3712–3717. [PubMed] [Google Scholar]
- Terajima J., Syuto B., Ochanda J. O., Kubo S. Purification and characterization of neurotoxin produced by Clostridium botulinum type C 6813. Infect Immun. 1985 May;48(2):312–317. doi: 10.1128/iai.48.2.312-317.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Ohishi I., Sakaguchi G. Fluid accumulation in mouse ligated intestine inoculated with Clostridium perfringens enterotoxin. Appl Environ Microbiol. 1979 Feb;37(2):181–186. doi: 10.1128/aem.37.2.181-186.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosawa N., Tsuzuki K., Syuto B., Oguma K. Activation of Clostridium botulinum type E toxin purified by two different procedures. J Gen Microbiol. 1986 Jul;132(7):1981–1988. doi: 10.1099/00221287-132-7-1981. [DOI] [PubMed] [Google Scholar]