Abstract
A set of phenethylamines has been successfully prepared via Suzuki-Miyaura cross-coupling of diverse potassium β-aminoethyltrifluoroborates with aryl halides. The potassium β-aminoethyltrifluoroborates were easily prepared via hydroboration of enamine and enamide precursors.
Phenethylamines and their structural analogues comprise important substructures of a variety of biologically important compounds including dopamine, tyrosine, amphetamine, and adrenaline. These privileged scaffolds are also widely found as components of alkaloid natural products1 and often serve as key building blocks in the synthesis of numerous nitrogen-containing complex molecules. Previous methods to introduce an aminoethyl group into an arene have employed the Friedel-Crafts acylation of activated arenes with N-protected amino acid chlorides,2 Heck arylation of N-vinyloxazolone followed by hydrogenation,3 and cross-coupling reactions involving β-amino organozinc reagents, which are somewhat unstable to β-elimination.4 Earlier investigations to access these important units also include the direct coupling of β-aminoethyl organolithiums with aryl- and alkenyl halides.5 A more broadly applicable Suzuki cross-coupling6 approach to this interesting class of compounds has been developed by Overman.7 However, although there are distinct advantages of this one-pot β-aminoethylation procedure in cross-coupling reactions and total synthesis (e.g., the reactions can be performed at room temperature),8 there are some limitations as well. In particular, the organoborane reagents prepared in situ via hydroboration of benzyl vinyl carbamate cannot be easily isolated and stored, but must be prepared and utilized on a reaction-by-reaction basis.
By contrast, potassium organotrifluoroborates have been shown to overcome this particular limitation. These salts are unique organoboron compounds, notable for their stability to moisture and air.9 They are powders or crystalline solids that are easy to access and handle, and these properties have made them attractive synthetic intermediates. Herein we describe our initial efforts to develop a convenient and practical access to phenethylamines via Suzuki cross-coupling of potassium β-aminoethyltrifluoroborates with aryl electrophiles.
To initiate studies on the aminoethylation reactions, a hydroboration protocol was employed that mimicked procedures reported by Overman.8 Thus, the respective N-vinyl substrates10 were hydroborated using Snieckus’ di(isopropylprenyl)borane (i-PP2BH),11 and the resulting organoborane intermediates were treated with an aqueous solution of KHF2 to afford the desired β-aminoethyltrifluoroborates 1a-e as depicted in Table 1.
Table 1.
Preparation of Potassium β-Aminoethyltrifluoroborates
 | |||
|---|---|---|---|
| entry | substrate | product | 1, % isolated yield |
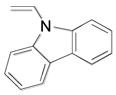
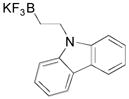
| 1 |
|
|
1a, 94 |
| 2 |
|
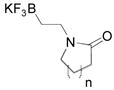
|
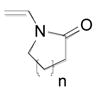
1b, n = 3; 76 |
| 3 | 1c, n = 1; 68 | ||
| 4 |
|
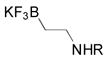
|
1d, R = Boc; 52 |
| 5 | 1e, R = Cbz; 50 | ||
Although organotrifluoroborate 1a has been obtained in excellent yield (Table 1, entry 1), the organotrifluoroborates containing a 7-membered and 5-membered lactam 1b and 1c (Table 1, entries 2–3) were obtained in lower yields. The Snieckus hydroboration protocol also tolerates nitrogen protecting groups as Boc and Cbz. However, the desired salts 1d and 1e were obtained in moderate yields (Table 1, entries 4–5). All of the new potassium β-aminoethyltrifluoroborates were obtained as powders or crystalline white solids that were stored on the benchtop without detectable degradation.
Initially, we focused on optimization of the reaction conditions for the Suzuki cross-coupling of β-aminoethyltrifluoroborates and aryl electrophiles (Table 2). Thus, 1a and 4-bromobenzonitrile were chosen as representative coupling partners. Based on the optimized cross-coupling reaction conditions reported between alkyltrifluoroborates and aryl bromides,12 we first conducted the reaction in the presence of different loadings of PdCl2(dppf)·CH2Cl2 using Cs2CO3 (3 equiv) as base, in a mixture of toluene/H2O (3:1) as solvent (Table 2, entries 1–3). It was observed that using 5 mol % of the catalyst, the desired phenethylamine 2a was obtained in 90% yield (Table 2, entry 2). Subsequently, systems were tested using 5 mol % of different palladium sources and ligands (Table 2, entries 4–7), but the desired product was formed in lower yields. The efficiency of PdCl2(dppf)·CH2Cl2 was also evaluated in the presence of different inorganic bases and Et3N, as well as in a different solvent [THF/H2O (10:1)]. However, in all cases no improvement could be observed (Table 2, entries 8–11).
Table 2.
Optimization of Cross-Coupling Reaction for the Synthesis of 2a
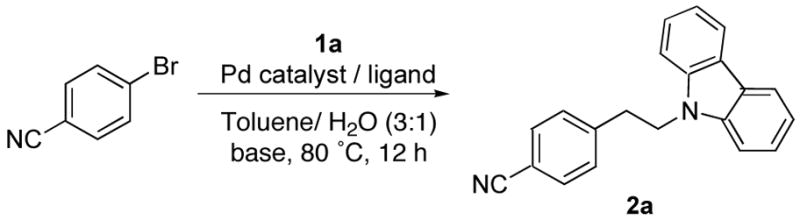 | |||
|---|---|---|---|
| entry | catalyst/ligand (mol %) | basea | % isolated yield |
| 1 | PdCl2(dppf)·CH2Cl2 (10) | Cs2CO3 | 84 |
| 2 | PdCl2(dppf)·CH2Cl2 (5) | Cs2CO3 | 90 |
| 3 | PdCl2(dppf)·CH2Cl2 (2) | Cs2CO3 | 68 |
| 4 | Pd(OAc)2/2 PPh3 (5) | Cs2CO3 | 78 |
| 5 | PdCl2/2 PPh3 (5) | Cs2CO3 | 56 |
| 6 | PdCl2(PPh3)2 (5) | Cs2CO3 | 72 |
| 7 | Pd(PPh3)4 (5) | Cs2CO3 | 51 |
| 8 | PdCl2(dppf)·CH2Cl2 (5) | K2CO3 | 69 |
| 9 | PdCl2(dppf)·CH2Cl2 (5) | K3PO4 | 77 |
| 10 | PdCl2(dppf)·CH2Cl2 (5) | Et3N | 73 |
| 11 | PdCl2(dppf)·CH2Cl2 (5) | Cs2CO3 | 75b |
3 equiv
A mixture of THF/H2O (10:1) was used as the solvent.
The optimized conditions were subsequently applied to cross-coupling reactions with electron-poor electrophiles, as depicted in Table 3. The reaction proceeds with comparable yields when different leaving groups attached to p-AcPhX (X = Br, I, OTf) were tested in the cross-coupling reaction (Table 3, entry 2). All of the desired phenethylamines were obtained with good to excellent yields (73–90%) in the presence of a wide variety of functional groups including nitriles, ketones, esters, aldehydes, halides, and nitro groups in the para position. The cross-coupling reaction also tolerates bromides with substituents in the ortho and meta positions, as the corresponding products were afforded in uniform yields (Table 3, entries 9–10).
Table 3.
Cross-Coupling of Potassium β-Aminoethyltrifluoroborate 1a with Electron-Poor Aryl Bromides
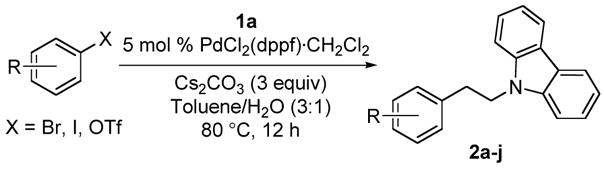 | ||
|---|---|---|
| entry | Ar-X | % isolated yield |
| 1 |
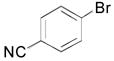
|
2a, 90 |
| 2 |
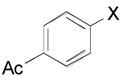
|
2b, X = Br, 82 |
| X = I, 79 | ||
| X = Otf, 77 | ||
| 3 |
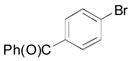
|
2c, 79 |
| 4 |
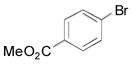
|
2d, 84 |
| 5 |
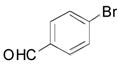
|
2e, 84 |
| 6 |
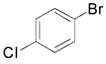
|
2f, 73 |
| 7 |
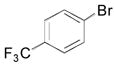
|
2g, 74 |
| 8 |
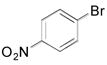
|
2h, 79 |
| 9 |
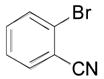
|
2i, 85 |
| 10 |
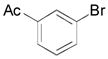
|
2j, 79 |
The introduction of an ethylamine moiety was also evaluated under the same reaction conditions using electron-rich bromides, as shown in Table 4. The coupling products were synthesized in moderate to good yields in the presence of functionalized bromides. Interestingly, comparable yields for the corresponding phenethylamines were obtained when a non-substituted bromide and a sterically hindered bromide were used as coupling partners (Table 4, compare entries 1 and 2). As observed with the electron-poor bromides, substituents located at different positions about the aromatic ring do not seem to show a strong influence in terms of yield for the respective phenethylamines (Table 4, entries 6 and 7).
Table 4.
Cross-Coupling of Potassium β-Aminoethyltrifluoroborate 1a with Electron-Rich Bromides
 | ||
|---|---|---|
| entry | Ar-Br | % isolated yield |
| 1 |
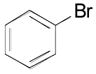
|
2k, 89 |
| 2 |
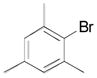
|
2l, 88 |
| 3 |
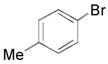
|
2m, 81 |
| 4 |
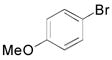
|
2n, 70 |
| 5 |
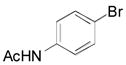
|
2o, 73 |
| 6 |
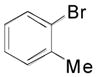
|
2p, 79 |
| 7 |
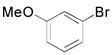
|
2q, 68 |
Next, the scope of the cross-coupling reaction was explored using heteroaryl bromides as coupling partners, as outlined in Table 5. The optimized conditions are quite general, as the present reaction provides the desired products in acceptable yields (Table 5, entries 1–3) and also tolerates different functional groups attached to furan, pyridine, and indole moieties. However, when a thiophene derivative was used, no coupling was observed. This unsatisfactory result prompted us to screen different coupling conditions as well as alternative ligands such as SPhos, XPhos and RuPhos (Figure 1).13
Table 5.
Cross-Coupling of Potassium β-Aminoethyltrifluoroborate 1a with Heteroaryl Bromides
 | ||
|---|---|---|
| entry | bromide | % isolated yield |
| 1 |
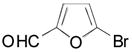
|
2r, 62 |
| 2 |
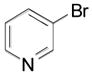
|
2s, 69 |
| 3 |
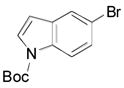
|
2t, 63 |
| 4 |
|
2u, 71a |
Pd(OAc)2 (2 mol %) and RuPhos (4 mol %) were used in the catalytic system.
Figure 1.
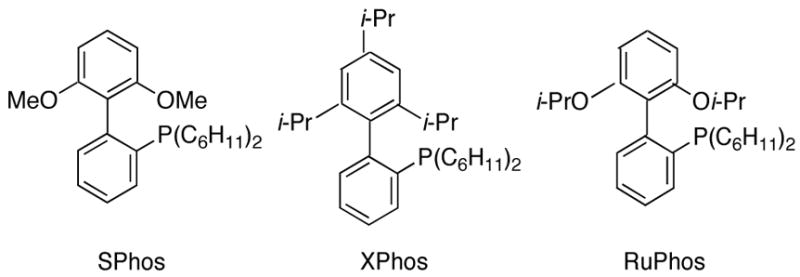
Buchwald Ligands
After investigating several conditions, 2 mol % of Pd(OAc)2 and 4 mol % of RuPhos (Table 5, entry 4) showed the best catalytic performance, affording the product in 71% yield.
Having demonstrated the efficiency of organotrifluoroborate 1a as a convenient aminoethylating agent for aryl- and heteroaryl bromides, we investigated the scope of the method using the β-aminoethyltrifluoroborates 1b and 1 c. We initially conducted the reaction using 5 mol % of PdCl2(dppf)·CH2Cl2, but the desired products were not obtained. Based on the successful procedure with the thiophene derivative, we used the RuPhos catalytic system, and in this case the phenethylamines 3a and 3b were obtained in 78% and 71% yields, respectively, as displayed in eq 1.
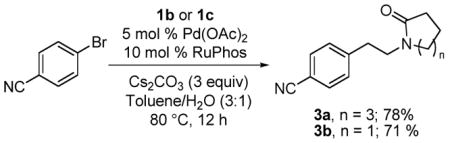 |
(1) |
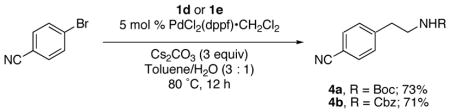
The organotrifluoroborates 1d and 1 e were also employed as coupling partners in the cross-coupling reaction. The previously optimized conditions were used to couple the corresponding β-aminoethyltrifluoroborates with 4-bromobenzonitrile, affording the corresponding phenethylamines 4a and 4b in good yields as outlined in eq 2. This coupling is particularly relevant because it provides an easy and efficient alternative to prepare phenethylamines containing free amino groups after appropriate deprotection of the respective Boc and Cbz protecting groups.
 |
(2) |
In summary, we have been described an efficient and convenient synthesis of an important class of compounds through the Suzuki-Miyaura cross-coupling reaction. The potassium β-aminoethyltrifluoroborates, readily obtained as stable crystalline solids in moderate to excellent yields via Snieckus hydroboration with i-PP2BH, have successfully proven their utility and versatility as useful aminoethylating reagents in the synthesis of a series of phenethylamines. The β-aminoethylation procedure tolerates diverse functional groups in the organotrifluoroborates as well as in the respective aryl-and heteroaryl electrophiles, affording the desired products in good to excellent yields. The stability of the β-aminoethyltrifluoroborates recommends them to diversity oriented synthesis, as a variety of these reagents can be prepared and stored indefinitely, awaiting coupling to diverse electrophiles. In this regard, studies directed toward a general method to prepare a wider range of potassium β-aminoethyltrifluoroborates are underway.
Supplementary Material
Experimental procedures, spectral characterization, and copies of 1H, 13C, 11B, and 19F spectra for all compounds. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (SWE 201094/2005-3). We thank the National Institutes of Health (GM 35249), Amgen, Merck Research Laboratories, and Johnson Matthey for their generous support.
References
- 1.(a) Bentley KW. Nat Prod Rep. 1999;16:367. [Google Scholar]; (b) Lednicer Mitscher LA. The Organic Chemistry of Drug Synthesis. Vol. 7 Wiley; New York: 1997. [Google Scholar]
- 2.Nordlander JE, Payne MJ, Njoroge FG, Balk MA, Laikos GD, Vishwanath VM. J Org Chem. 1984;49:4107. [Google Scholar]
- 3.Busacca CA, Johnson RE, Swestock J. J Org Chem. 1993;58:3299. [Google Scholar]
- 4.(a) Duddu R, Eckhardt M, Furlong M, Knoess HP, Berger S, Knochel P. Tetrahedron. 1994;50:2415. [Google Scholar]; (b) Hunter C, Jackson RFW, Rami HK. J Chem Soc, Perkin Trans 1. 2000:219. [Google Scholar]; (c) Rilatt I, Caggiano L, Jackson RFW. Synlett. 2005:2701. [Google Scholar]
- 5.Barluenga J, Montserrat JM, Florez J. J Org Chem. 1993;58:5976. [Google Scholar]
- 6.For a review of the Suzuki reaction, see: Miyaura N, Suzuki A. Chem Rev. 1995;95:2457.Kotha S, Lahiri K, Dhurke K. Tetrahedron. 2002;58:9633.
- 7.Kamatani A, Overman LE. J Org Chem. 1999;64:8743. [Google Scholar]
- 8.(a) Kamatani A, Overman LE. Org Lett. 2001;3:1229. doi: 10.1021/ol015696v. [DOI] [PubMed] [Google Scholar]; (b) Dounay AB, Overman LE, Wrobleski AD. J Am Chem Soc. 2005;127:10186. doi: 10.1021/ja0533895. [DOI] [PubMed] [Google Scholar]; (c) Fuchs JR, Funk RL. Org Lett. 2005;7:677. doi: 10.1021/ol047532v. [DOI] [PubMed] [Google Scholar]
- 9.For the synthesis of organotrifluoroborates see: Vedejs E, Chapman RW, Fields SC, Liu S, Schrimpf MR. J Org Chem. 1999;60:3020.Vedejs E, Fields SC, Hayashi R, Hitchcock SR, Powell DR, Schrimpf MR. J Am Chem Soc. 1999;121:2460. For reviews of organotrifluoroborate salts, see: Molander GA, Figueroa R. Aldrichimica Acta. 2005;38:49.Darses S, Genêt JP. Eur J Org Chem. 2003:4313.Molander GA, Ellis N. Acc Chem Res. doi: 10.1021/ar050199q. in press.
- 10.9-Vinylcarbazole, N -vinylcaprolactam and N -vinyl-2-pyrrolidone are commercially available; N-Boc vinyl carbamate and N-Cbz vinyl carbamate were prepared according to the procedures described in the literature (See Supporting Information).
- 11.Kalinin AV, Scherer S, Snieckus V. Angew Chem Int Ed. 2003;42:3399. doi: 10.1002/anie.200351312. [DOI] [PubMed] [Google Scholar]
- 12.(a) Molander GA, Ito T. Org Lett. 2001;3:393. doi: 10.1021/ol006896u. [DOI] [PubMed] [Google Scholar]; (b) Molander GA, Yun CS, Ribagorda M, Biolatto B. J Org Chem. 2003;68:5534. doi: 10.1021/jo0343331. [DOI] [PubMed] [Google Scholar]
- 13.(a) Milne JE, Buchwald SL. J Am Chem Soc. 2004;126:13028. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]; (b) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, spectral characterization, and copies of 1H, 13C, 11B, and 19F spectra for all compounds. This material is available free of charge via the internet at http://pubs.acs.org.


