Abstract
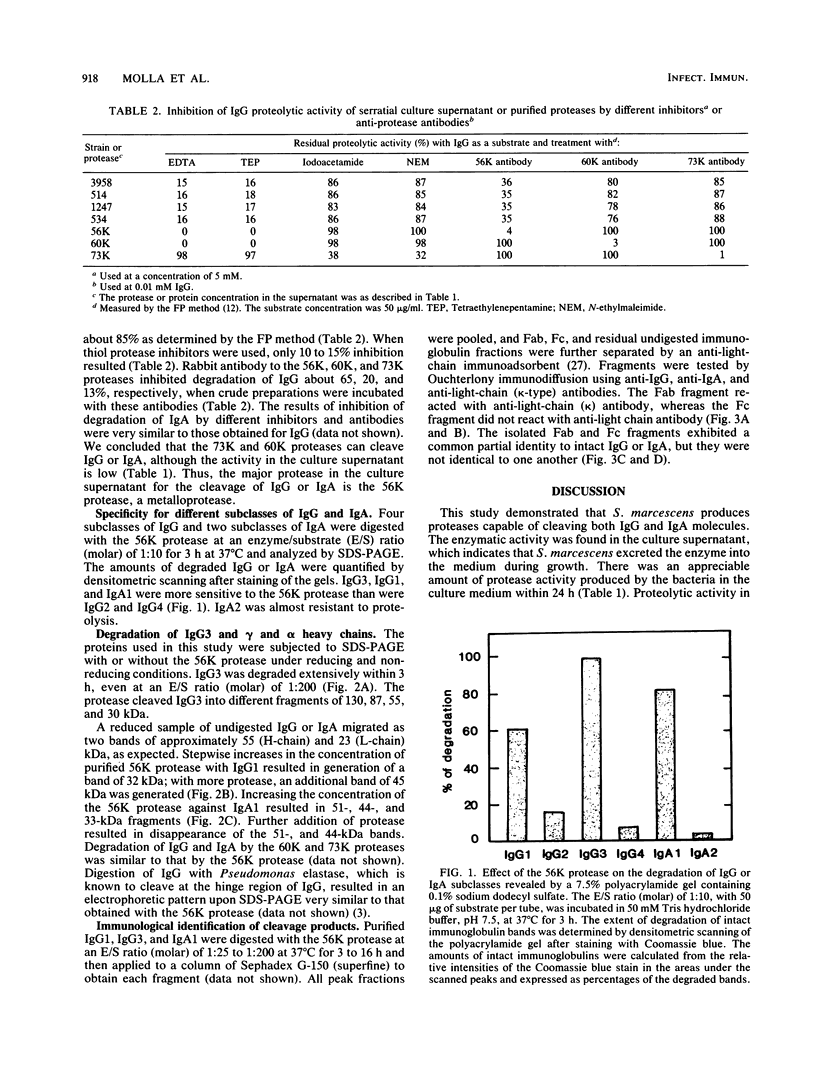
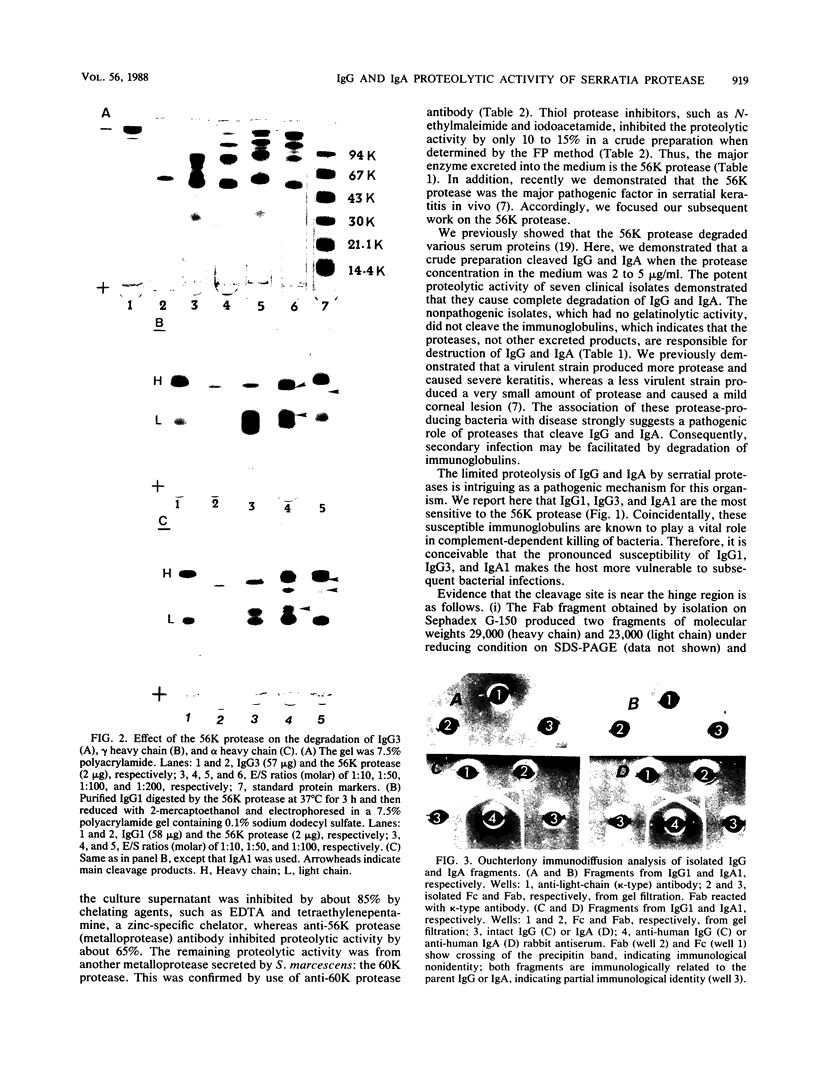
Seven clinical and two nonclinical isolates of Serratia marcescens were examined for their ability to produce extracellular enzymes that cleave immunoglobulin G (IgG) and IgA molecules. All seven clinical isolates excreted a large amount of a 56-kilodalton (kDa) protease (56K protease) and small amounts of a 60-kDa and a 73-kDa protease (60K and 73K proteases, respectively) in culture medium during growth. All purified proteases cleaved IgG and IgA effectively if the level of protease production exceeded 2 to 5 micrograms/ml. The proteolytic activity in the culture supernatant was inhibited by about 85% by a chelating agent (EDTA), which indicated that the major immunoglobulin-cleaving enzyme is the metalloprotease(s) reported previously. Immunological quantification of proteases by single radial immunodiffusion showed similar results: the amount of 56K protease was about 65% and those of the 60K and 73K proteases were about 20 and 5%, respectively. Incubation for 3 h at 37 degrees C was required to generate immunoreactive Fab and Fc fragments. Further analysis of the cleavage products of IgG or IgA demonstrated that the 56K protease, as well as the 60K and 73K proteases, cleaves only the heavy chain of these immunoglobulins near the hinge region to generate Fab and Fc fragments. The susceptibilities of the subclasses of IgG and IgA to the 56K protease were as follows: IgG3 greater than IgG1 greater than IgG2 greater than IgG4 and IgA1 greater than IgA2. IgG2, IgG4, and IgA2 were relatively resistant to the 56K protease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doellgast G. J., Plaut A. G. Purification of human IgA by salt-mediated hydrophobic chromatography. Immunochemistry. 1976 Feb;13(2):135–139. doi: 10.1016/0019-2791(76)90281-0. [DOI] [PubMed] [Google Scholar]
- Duhamel R. C., Schur P. H., Brendel K., Meezan E. pH gradient elution of human IgG1, IgG2 and IgG4 from protein A-sepharose. J Immunol Methods. 1979;31(3-4):211–217. doi: 10.1016/0022-1759(79)90133-9. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Baltimore R. S., Squier S. U., Reynolds H. Y. IgG proteolytic activity of Pseudomonas aeruginosa in cystic fibrosis. J Infect Dis. 1985 Apr;151(4):589–598. doi: 10.1093/infdis/151.4.589. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Reynolds H. Y. Use of Pseudomonas aeruginosa lipopolysaccharide immunoadsorbents to prepare high potency, mono-specific antibodies. J Immunol Methods. 1980;38(1-2):103–116. doi: 10.1016/0022-1759(80)90335-x. [DOI] [PubMed] [Google Scholar]
- Kamata R., Matsumoto K., Okamura R., Yamamoto T., Maeda H. The serratial 56K protease as a major pathogenic factor in serratial keratitis. Clinical and experimental study. Ophthalmology. 1985 Oct;92(10):1452–1459. doi: 10.1016/s0161-6420(85)33855-1. [DOI] [PubMed] [Google Scholar]
- Kamata R., Yamamoto T., Matsumoto K., Maeda H. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs. Infect Immun. 1985 Jun;48(3):747–753. doi: 10.1128/iai.48.3.747-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Kreger A. S. Importance of serratia protease in the pathogenesis of experimental Serratia marcescens pneumonia. Infect Immun. 1983 Apr;40(1):113–119. doi: 10.1128/iai.40.1.113-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D., Gray L., Kreger A. Characterization of rabbit corneal damage produced by Serratia keratitis and by a serratia protease. Infect Immun. 1981 Sep;33(3):927–932. doi: 10.1128/iai.33.3.927-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D., Kreger A. Purification and characterization of a Serratia marcescens metalloprotease. Infect Immun. 1979 May;24(2):411–421. doi: 10.1128/iai.24.2.411-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. Assay of proteolytic enzymes by the fluorescence polarization technique. Anal Biochem. 1979 Jan 1;92(1):222–227. doi: 10.1016/0003-2697(79)90649-3. [DOI] [PubMed] [Google Scholar]
- Maeda H., Molla A., Oda T., Katsuki T. Internalization of serratial protease into cells as an enzyme-inhibitor complex with alpha 2-macroglobulin and regeneration of protease activity and cytotoxicity. J Biol Chem. 1987 Aug 15;262(23):10946–10950. [PubMed] [Google Scholar]
- Maki D. G., Hennekens C. G., Phillips C. W., Shaw W. V., Bennett J. V. Nosocomial urinary tract infection with Serratia marcescens: an epidemiologic study. J Infect Dis. 1973 Nov;128(5):579–587. doi: 10.1093/infdis/128.5.579. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Maeda H., Okamura R. Role of the zinc atom in the activity and conformational stability of serratial 56K protease. A fluorometric study. J Biochem. 1984 Oct;96(4):1165–1173. doi: 10.1093/oxfordjournals.jbchem.a134934. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Maeda H., Takata K., Kamata R., Okamura R. Purification and characterization of four proteases from a clinical isolate of Serratia marcescens kums 3958. J Bacteriol. 1984 Jan;157(1):225–232. doi: 10.1128/jb.157.1.225-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984 Sep;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- Molla A., Matsumoto K., Oyamada I., Katsuki T., Maeda H. Degradation of protease inhibitors, immunoglobulins, and other serum proteins by Serratia protease and its toxicity to fibroblast in culture. Infect Immun. 1986 Sep;53(3):522–529. doi: 10.1128/iai.53.3.522-529.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Matsumura Y., Yamamoto T., Okamura R., Maeda H. Pathogenic capacity of proteases from Serratia marcescens and Pseudomonas aeruginosa and their suppression by chicken egg white ovomacroglobulin. Infect Immun. 1987 Oct;55(10):2509–2517. doi: 10.1128/iai.55.10.2509-2517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Oda T., Maeda H. Different binding kinetics of Serratia 56K protease with plasma alpha 2-macroglobulin and chicken egg white ovomacroglobulin. J Biochem. 1987 Jan;101(1):199–205. doi: 10.1093/oxfordjournals.jbchem.a121892. [DOI] [PubMed] [Google Scholar]
- Old D. C., Adegbola R., Scott S. S. Multiple fimbrial haemagglutinins in Serratia species. Med Microbiol Immunol. 1983;172(2):107–115. doi: 10.1007/BF02124511. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Brown T. A., Mestecky J. Role of serum IgA. Hepatobiliary transport of circulating antigen. J Exp Med. 1981 Apr 1;153(4):968–976. doi: 10.1084/jem.153.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvaril F., Morell A. The G4 subclass in IgG fractions prepared by ion-exchange chromatography. J Immunol. 1970 May;104(5):1310–1312. [PubMed] [Google Scholar]
- Virca G. D., Lyerly D., Kreger A., Travis J. Inactivation of human plasma alpha 1-proteinase inhibitor by a metalloproteinase from Serratia marcescens. Biochim Biophys Acta. 1982 Jun 4;704(2):267–271. doi: 10.1016/0167-4838(82)90155-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilchek M., Bocchini V., Becker M., Givol D. A general method for the specific isolation of peptides containing modified residues, using insoluble antibody columns. Biochemistry. 1971 Jul 20;10(15):2828–2834. doi: 10.1021/bi00791a004. [DOI] [PubMed] [Google Scholar]
- Yonemura K., Matsumoto K., Maeda H. Isolation and characterization of nucleases from a clinical isolate of Serratia marcescens kums 3958. J Biochem. 1983 May;93(5):1287–1295. doi: 10.1093/oxfordjournals.jbchem.a134262. [DOI] [PubMed] [Google Scholar]