Abstract
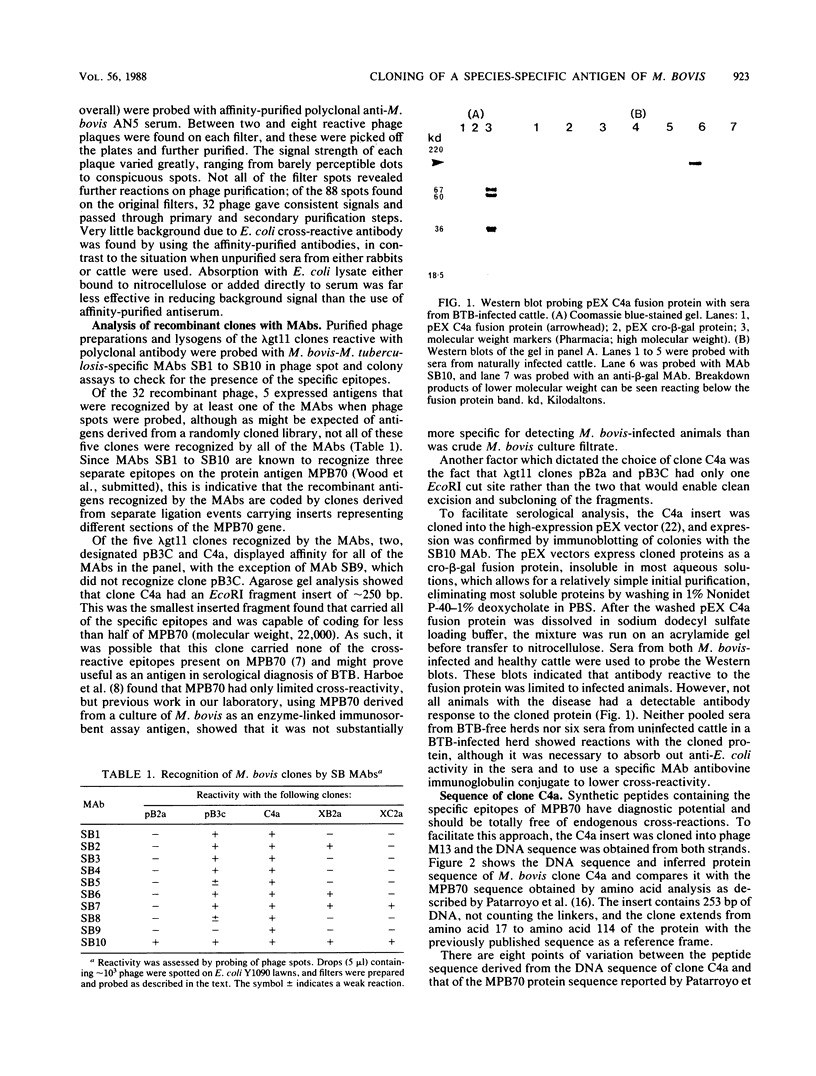
A DNA library from a virulent strain of Mycobacterium bovis was constructed in the expression vector lambda gt11, and the library was probed with antisera to M. bovis. Clones expressing M. bovis antigens were isolated and characterized by using M. bovis-specific monoclonal antibodies that recognize a 22,000-molecular-weight protein (MPB70). MPB70 is a major protein antigen of the vaccine strain of M. bovis BCG and of virulent M. bovis, the causative agent of bovine tuberculosis. Of 32 clones selected by using polyclonal affinity-purified anti-M. bovis sera, 5 were recognized by the anti-MPB-70 monoclonal antibodies, and one monoclonal antibody, SB10, recognized all 5 clones. Characterization of these clones showed that one clone containing a 253-base-pair insert expressed a polypeptide bound by all of the MPB70-specific monoclonal antibodies. Western blots (immunoblots) showed that this cloned protein was recognized by sera from M. bovis-infected cattle, although not all cattle with bovine tuberculosis produced antibodies reactive to this clone. DNA sequencing of the clone showed that it coded for 84 amino acids from positions 17 to 114 of the 161-amino-acid protein, with a 16-peptide deletion between positions 79 and 94. Apart from this deletion, there were seven other variations between the cloned sequence and that deduced from M. bovis BCG MPB70.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Smith I., Grange J., Steele J., Rook G. Subdivision of daughter strains of bacille Calmette-Guérin (BCG) according to secreted protein patterns. J Gen Microbiol. 1986 Nov;132(11):3047–3053. doi: 10.1099/00221287-132-11-3047. [DOI] [PubMed] [Google Scholar]
- Auer L. A. Assessment of an enzyme linked immunosorbent assay for the detection of cattle infected with Mycobacterium bovis. Aust Vet J. 1987 Jun;64(6):172–176. doi: 10.1111/j.1751-0813.1987.tb09676.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984 Mar;129(3):444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- Harboe M., Nagai S., Patarroyo M. E., Torres M. L., Ramirez C., Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986 Apr;52(1):293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Nagai S., Kinomoto M., Haga S., Tokunaga T. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect Immun. 1983 Feb;39(2):540–545. doi: 10.1128/iai.39.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Matsumoto J., Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981 Mar;31(3):1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- PATERSON A. B., STUART P., LESSLIE I. W. The use of tests on slaughterhouse cattle for estimating relative potencies of tuberculins and for the calculation of discrimination tests. J Hyg (Lond) 1958 Mar;56(1):1–18. doi: 10.1017/s0022172400037505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritacco V., de Kantor I. N., Barrera L., Nader A., Bernardelli A., Torrea G., Errico F., Fliess E. Assessment of the sensitivity and specificity of enzyme-linked immunosorbent assay (ELISA) for the detection of mycobacterial antibodies in bovine tuberculosis. Zentralbl Veterinarmed B. 1987 Mar;34(2):119–125. doi: 10.1111/j.1439-0450.1987.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. A., Fisher J. H., Jones W. D., Jr, Scoggin C. H. Restriction fragment analysis of chromosomal DNA defines different strains of Mycobacterium tuberculosis. Am Rev Respir Dis. 1986 Aug;134(2):210–213. doi: 10.1164/arrd.1986.134.2.210. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]



