Abstract
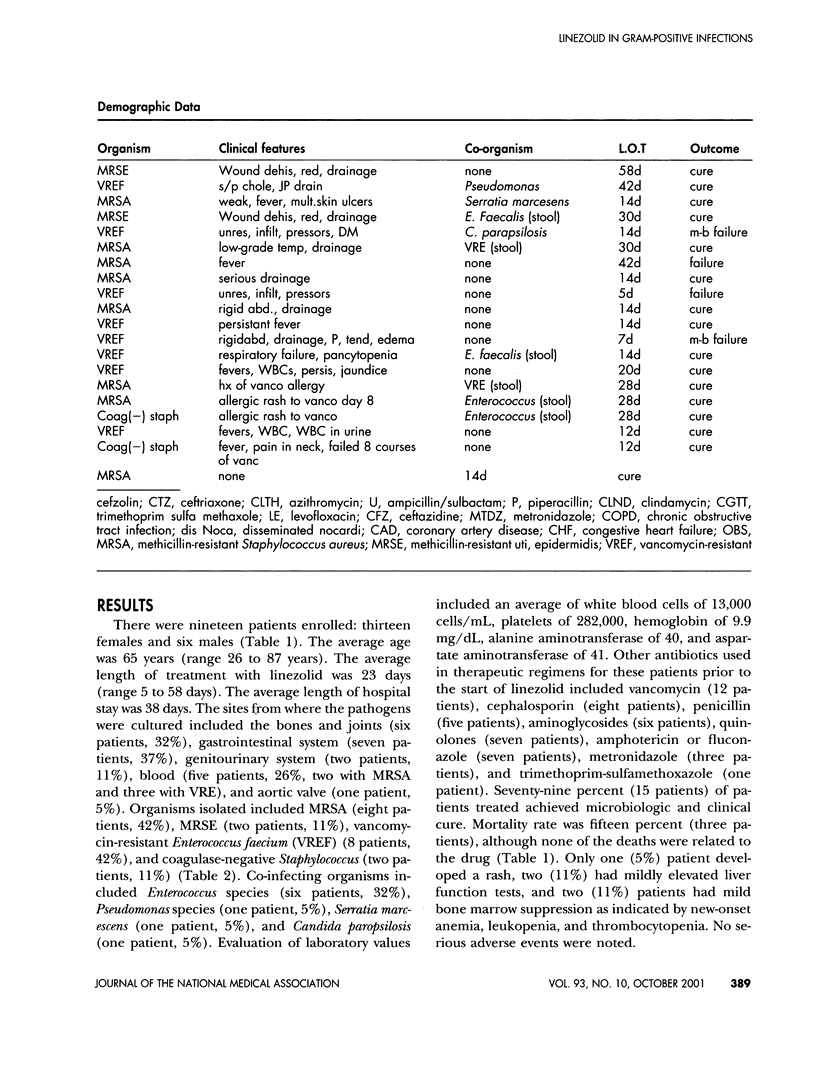
This study presents our clinical experience with linezolid in 19 patients with serious resistant gram-positive infections enrolled as part of the compassionate study. In this prospective, non-randomized, noncomparative study, 19 patients were enrolled as part of the National Compassionate Study Protocol conducted by Pharmacia-Upjohn. At the time of this writing, these patients had not been published in the literature. All of the patients had to have documented evidence of serious gram-positive infections in normally sterile sites and should have been unable to tolerate available antimicrobial therapy or be unresponsive to available drugs. Clinical characteristics, laboratory values, and pharmacokinetic and pharmacodynamic parameters were obtained. Patients were followed both short-term and long-term after completion of therapy. Nineteen patients were enrolled: 13 females and 6 males. The average age was 63 years. The average length of therapy with linezolid was 22 days. Methicillin-resistant Staphylococcus aureus (MRSA) was treated in eight patients, methicillin-resistant Staphylococcus epidermidis (MRSE) in two patients, vancomycin-resistant Enterococcus faecium (VREF) in eight patients, and coagulase-negative Staphylococcus in two patients. Co-infecting organisms include Enterococcus species colonization in six patients, Pseudomonas species in one patient, Serratia marcenens in one patient, and Candida albicans in one patient. Sterile sites that were infected included bone and joint (wounds and septic joints) in six patients, gastrointestinal system (hepatobiliary, liver abscess, Crohn's) in five patients, genitourinary (kidney and urine) in two patients, blood in five patients, respiratory in one patient, and aortic valve in 1 patient. Linezolid was given at 600 mg IV every 12 hours with a mean length of therapy of 22 days. Surgical drainage was used in combination with linezolid in 11 of the patients. Seventy nine percent of these patients achieved clinical and microbiologic cure, and none of the deaths reported in this series were related to the drug. Adverse events included skin rash in one patient, mild bone marrow suppression in two patients, and mild elevation in liver function tests in two patients. No life-threatening adverse events were noted. It appears that linezolid, along with surgical intervention (when necessary), appears to be an effective treatment option for resistant gram-positive infections. Long-term studies evaluating the possible resistance rates are necessary.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony S. J., Bitter K. M., Moreland T., Raudales F., Diaz-Luna H. Methicillin-resistant Staphylococcus aureus infection in a renal allograft recipient treated successfully with a novel new antimicrobial agent (linezolid): new treatment options for infections due to resistant organisms. Clin Infect Dis. 1999 Nov;29(5):1341–1342. doi: 10.1086/313492. [DOI] [PubMed] [Google Scholar]
- Barry A. L. In vitro evaluation of DuP 105 and DuP 721, two new oxazolidinone antimicrobial agents. Antimicrob Agents Chemother. 1988 Jan;32(1):150–152. doi: 10.1128/aac.32.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostic G. D., Perri M. B., Thal L. A., Zervos M. J. Comparative in vitro and bactericidal activity of oxazolidinone antibiotics against multidrug-resistant enterococci. Diagn Microbiol Infect Dis. 1998 Feb;30(2):109–112. doi: 10.1016/s0732-8893(97)00210-1. [DOI] [PubMed] [Google Scholar]
- Chien J. W., Kucia M. L., Salata R. A. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin Infect Dis. 2000 Jan;30(1):146–151. doi: 10.1086/313597. [DOI] [PubMed] [Google Scholar]
- Daly J. S., Eliopoulos G. M., Reiszner E., Moellering R. C., Jr Activity and mechanism of action of DuP 105 and DuP 721, new oxazolidinone compounds. J Antimicrob Chemother. 1988 Jun;21(6):721–730. doi: 10.1093/jac/21.6.721. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C. B., Gold H. S., Moellering R. C., Jr In vitro activities in new oxazolidinone antimicrobial agents against enterococci. Antimicrob Agents Chemother. 1996 Jul;40(7):1745–1747. doi: 10.1128/aac.40.7.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Johnson D. M., Erwin M. E. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob Agents Chemother. 1996 Mar;40(3):720–726. doi: 10.1128/aac.40.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1996 Mar;40(3):799–801. doi: 10.1128/aac.40.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Novelli A., Saha G., Chin N. X. In vitro activities of two oxazolidinone antimicrobial agents, DuP 721 and DuP 105. Antimicrob Agents Chemother. 1988 Apr;32(4):580–583. doi: 10.1128/aac.32.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Rouse M. S., Piper K. E., Steckelberg J. M. In vitro activity of linezolid against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 1999 Jun;34(2):119–122. doi: 10.1016/s0732-8893(99)00016-4. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Wuonola M. A., McRipley R. J., Zajac I., Zawada M. J., Bartholomew P. T., Gregory W. A., Forbes M. Oxazolidinones, a new class of synthetic antibacterial agents: in vitro and in vivo activities of DuP 105 and DuP 721. Antimicrob Agents Chemother. 1987 Nov;31(11):1791–1797. doi: 10.1128/aac.31.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurenko G. E., Yagi B. H., Schaadt R. D., Allison J. W., Kilburn J. O., Glickman S. E., Hutchinson D. K., Barbachyn M. R., Brickner S. J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996 Apr;40(4):839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]


