Abstract
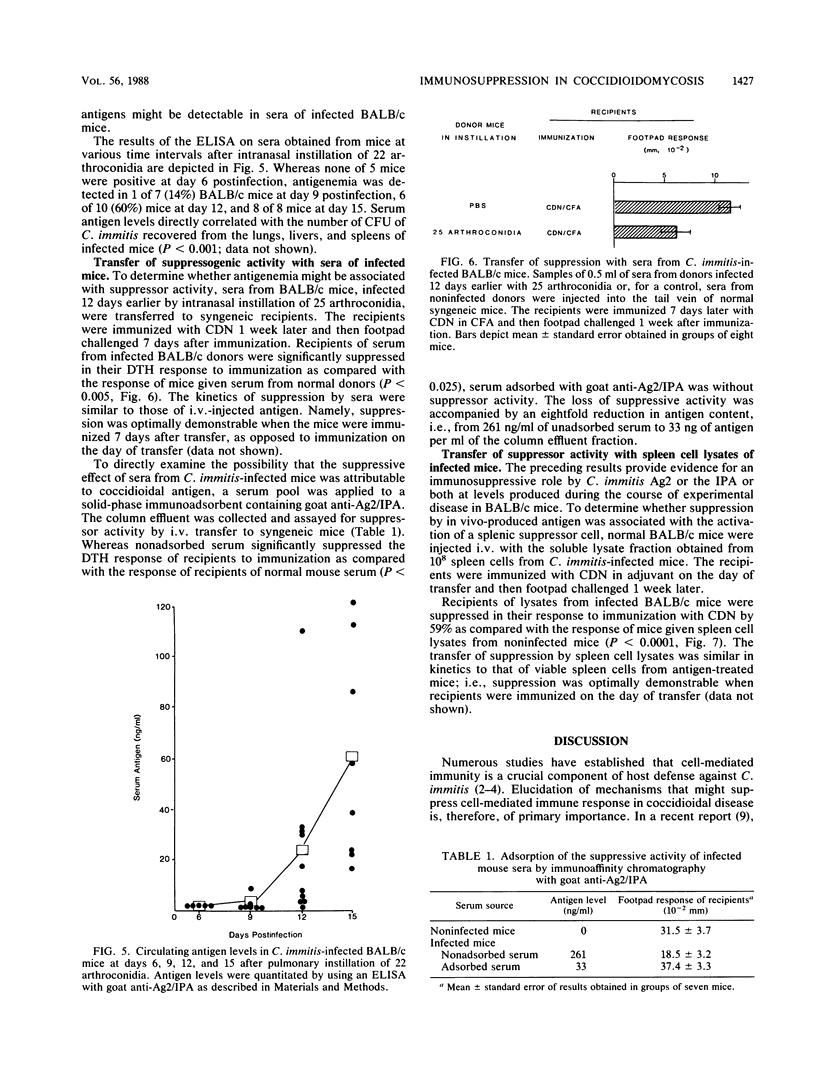
Intravenous injection of BALB/c mice with coccidioidin or an alkali-soluble cell wall extract of Coccidioides immitis mycelia resulted in the induction of a splenic cell population(s) that suppressed delayed-type hypersensitivity response to coccidioidal antigen. To determine whether the levels of C. immitis antigen produced during the course of active coccidioidal disease might also cause suppression of T-lymphocyte response, BALB/c mice were infected by intranasal instillation of arthroconidia, and 2 weeks later, their sera were evaluated for suppression of T-lymphocyte response in syngeneic recipients. Intravenous transfer of sera, which were shown to contain high levels of coccidioidal antigen by an enzyme-linked immunoadsorbent assay, suppressed the delayed-type hypersensitivity response of recipients to immunization with coccidioidin. Solid-phase immunoadsorption of the sera with goat antibodies to C. immitis antigens removed the suppressive component(s). To determine whether the suppressive effect of circulating coccidioidal antigen(s) was associated with the activation of a splenic suppressor cell(s), as was observed in mice injected intravenously with coccidioidal antigen, spleen cell lysates were prepared from infected donors, and after filtration to remove viable fungi, the lysates were transferred to syngeneic mice. Recipients of lysates from infected but not noninfected donors were suppressed in their response to immunization with coccidioidin. Collectively, these results provide evidence that depressed T-cell responses observed in coccidioidomycosis are associated with, and may be attributable to, the activation of a suppressor cell or factor by circulating C. immitis antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L. V., Pappagianis D., Benjamini E. Mechanisms of resistance to infection with Coccidioides immitis in mice. Infect Immun. 1979 Mar;23(3):681–685. doi: 10.1128/iai.23.3.681-685.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun. 1981 Nov;34(2):347–353. doi: 10.1128/iai.34.2.347-353.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow E. W., Domer J. E. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect Immun. 1985 Jul;49(1):172–181. doi: 10.1128/iai.49.1.172-181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro A., Spitler L. E., Moser K. M. Cellular immune response in coccidioidomycosis. Cell Immunol. 1975 Feb;15(2):360–371. doi: 10.1016/0008-8749(75)90014-3. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Britt L. A. Antigenic heterogeneity of an alkali-soluble, water-soluble cell wall extract of Coccidioides immitis. Infect Immun. 1985 Nov;50(2):365–369. doi: 10.1128/iai.50.2.365-369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Britt L. A. Isolation of a coccidioidin component that reacts with immunoglobulin M precipitin antibody. Infect Immun. 1986 Sep;53(3):449–453. doi: 10.1128/iai.53.3.449-453.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Kennell W., Boncyk L., Murphy J. W. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988 Jan;56(1):13–17. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Pope R. M., Stevens D. A. Immune complexes in coccidioidomycosis. Correlation with disease involvement. Am Rev Respir Dis. 1982 Sep;126(3):439–443. doi: 10.1164/arrd.1982.126.3.439. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R., Gross A., Lecara G., Miller E., Brummer E. In vivo and in vitro cell-mediated responses in coccidioidomycosis. I. Immumologic responses of persons with primary, asymptomatic infections. Am Rev Respir Dis. 1976 Nov;114(5):937–943. doi: 10.1164/arrd.1976.114.5.937. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell Immunol. 1977 Jun 1;31(1):130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Catanzaro A. Coccidioidomycosis. Part II. Am Rev Respir Dis. 1978 Apr;117(4):727–771. doi: 10.1164/arrd.1978.117.4.727. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Geha R. S. Idiotypic determinants on human T cells and modulation of human T cell responses by anti-idiotypic antibodies. J Immunol. 1984 Oct;133(4):1846–1851. [PubMed] [Google Scholar]
- Ibrahim A. B., Pappagianis D. Experimental induction of anergy to coccidioidin by antigens of Coccidioides immitis. Infect Immun. 1973 May;7(5):786–794. doi: 10.1128/iai.7.5.786-794.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Fierer J. Genetic control of resistance to Coccidioides immitis: a single gene that is expressed in spleen cells determines resistance. J Immunol. 1985 Jul;135(1):548–552. [PubMed] [Google Scholar]
- Kirkland T. N., Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun. 1983 Jun;40(3):912–916. doi: 10.1128/iai.40.3.912-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., KONG Y. C., SMITH C. IMMUNIZATION OF MICE TO COCCIDIOIDES IMMITIS: DOSE, REGIMEN AND SPHERULATION STAGE OF KILLED SPHERULE VACCINES. J Immunol. 1965 Jan;94:132–142. [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- Rivas V., Rogers T. J. Studies on the cellular nature of Candida albicans-induced suppression. J Immunol. 1983 Jan;130(1):376–379. [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., SIMONS S. A. Pattern of 39,500 serologic tests in coccidioidomycosis. J Am Med Assoc. 1956 Feb 18;160(7):546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., WHITING E. G. The use of coccidioidin. Am Rev Tuberc. 1948 Apr;57(4):330–360. doi: 10.1164/art.1948.57.4.330. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Bach B. A., Dohi Y., Nisonoff A., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. I. Induction of suppressor T cells with anti-idiotypic antibodies. J Exp Med. 1979 Nov 1;150(5):1216–1228. doi: 10.1084/jem.150.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinoya S., Cox R. A., Pope R. M. Circulating immune complexes in coccidioidomycosis. Detection and characterization. J Clin Invest. 1980 Oct;66(4):655–663. doi: 10.1172/JCI109901. [DOI] [PMC free article] [PubMed] [Google Scholar]


