Abstract
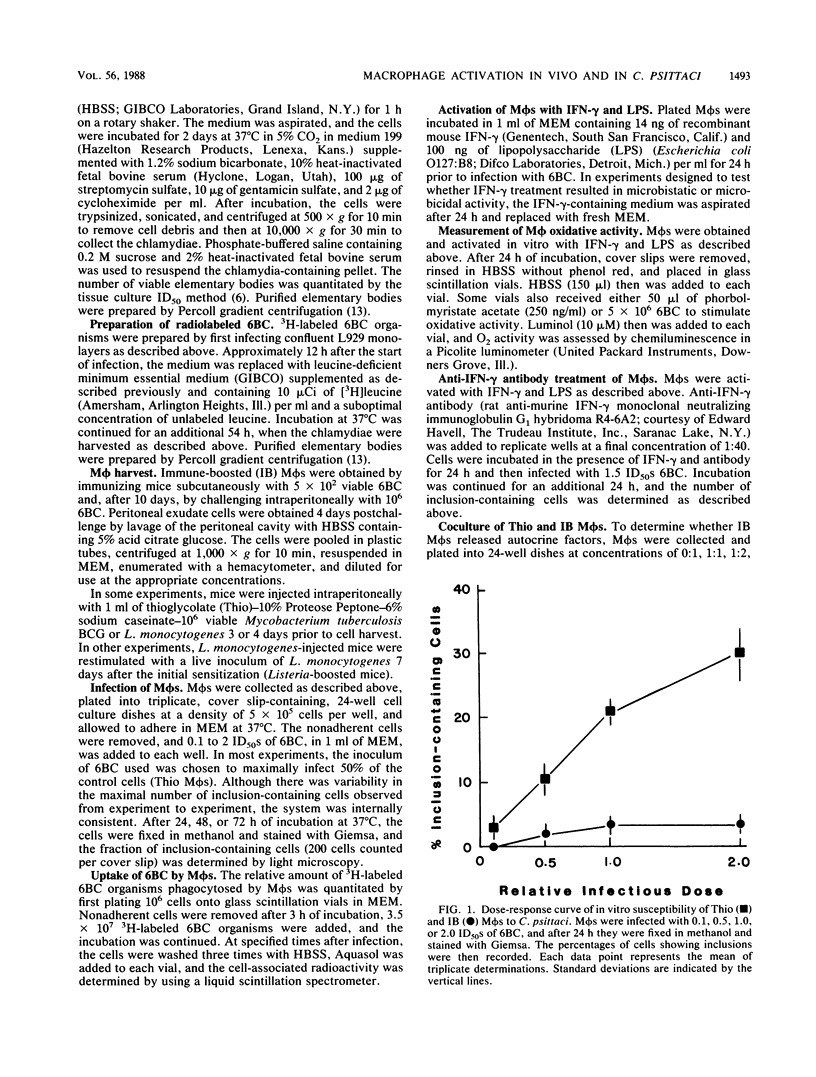
Peritoneal macrophages (M phi s) collected from Chlamydia psittaci 6BC-immune mice after intraperitoneal challenge with 10(6) 6BC (immune-boosted [IB] M phi s) were compared by various functional criteria with other in vivo- and in vitro-activated M phi populations. While casein-, protease peptone-, and thioglycolate (Thio)-elicited M phi s were equally susceptible to in vitro infection with 6BC, IB M phi s did not support chlamydial growth and M phi s from Mycobacterium tuberculosis BCG- or Listeria monocytogenes-sensitized mice exhibited intermediate susceptibility to infection. The resistance of IB M phi s was not due to the ingestion of fewer 6BC organisms, nor were these cells persistently infected, since chlamydiae could not be recovered from infected IB M phi s after in vitro infection, even after extended incubation times. In contrast, Thio M phi s stimulated in vitro with gamma interferon (IFN-gamma), with or without lipopolysaccharide, resulted in cells that exhibited chlamydiastatic activity which was lost shortly after IFN-gamma was removed from the culture medium. Conversely, the antichlamydial activity of IB M phi s was stable over time but not through the production of autostimulatory cytokines, as evidenced by the lack of stimulation of Thio M phi s to restrict 6BC replication in coculture experiments. IB M phi s exhibited enhanced oxidative activity, but anti-IFN-gamma antibody did not abrogate this response. IB M phi s were recovered only from immunized mice that survived an otherwise lethal 6BC intraperitoneal challenge. These cells appear to be important for development of protective immunity to chlamydiae, and evidence suggests that stimulation by cytokines other than IFN-gamma (with or without lipopolysaccharide) is required for the observed heightened in vivo activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Faubion C. L. Inhibition of Chlamydia psittaci in oxidatively active thioglycolate-elicited macrophages: distinction between lymphokine-mediated oxygen-dependent and oxygen-independent macrophage activation. Infect Immun. 1983 May;40(2):464–471. doi: 10.1128/iai.40.2.464-471.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R., Suntharalingam K., Fikrig S. The effect of Chlamydia trachomatis on luminol-dependent chemiluminescence of human polymorphonuclear leukocytes: requirements for opsonization. J Infect Dis. 1985 Jun;151(6):1045–1051. doi: 10.1093/infdis/151.6.1045. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Finbloom D. S., Crawford R. M., Nacy C. A., Gilbreath M., Meltzer M. S. A lymphokine distinct from interferon-gamma that activates human monocytes to kill Leishmania donovani in vitro. J Immunol. 1986 Feb 15;136(4):1329–1333. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Kitagawa S. Molecular basis for the enhanced respiratory burst of activated macrophages. Fed Proc. 1985 Nov;44(14):2927–2932. [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Mononuclear phagocyte antimicrobial and antitumor activity: the role of oxygen intermediates. J Invest Dermatol. 1980 May;74(5):285–288. doi: 10.1111/1523-1747.ep12543457. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Schachter J., Grossman M. Chlamydial infections. Annu Rev Med. 1981;32:45–61. doi: 10.1146/annurev.me.32.020181.000401. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A. Growth of Chlamydia psittaci in macrophages. Infect Immun. 1978 Mar;19(3):1054–1060. doi: 10.1128/iai.19.3.1054-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Klebanoff S. J., Kuo C. C. Toxic effect of human polymorphonuclear leukocytes on Chlamydia trachomatis. Infect Immun. 1982 Aug;37(2):422–426. doi: 10.1128/iai.37.2.422-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


