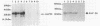Abstract
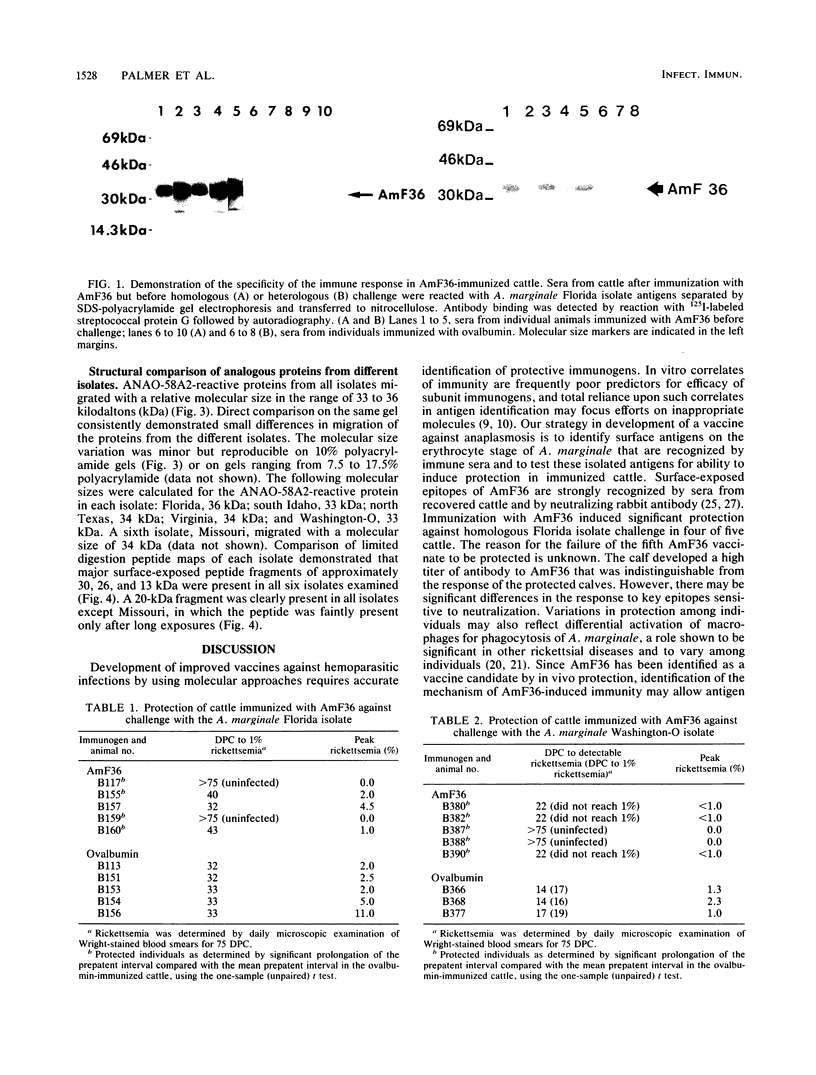
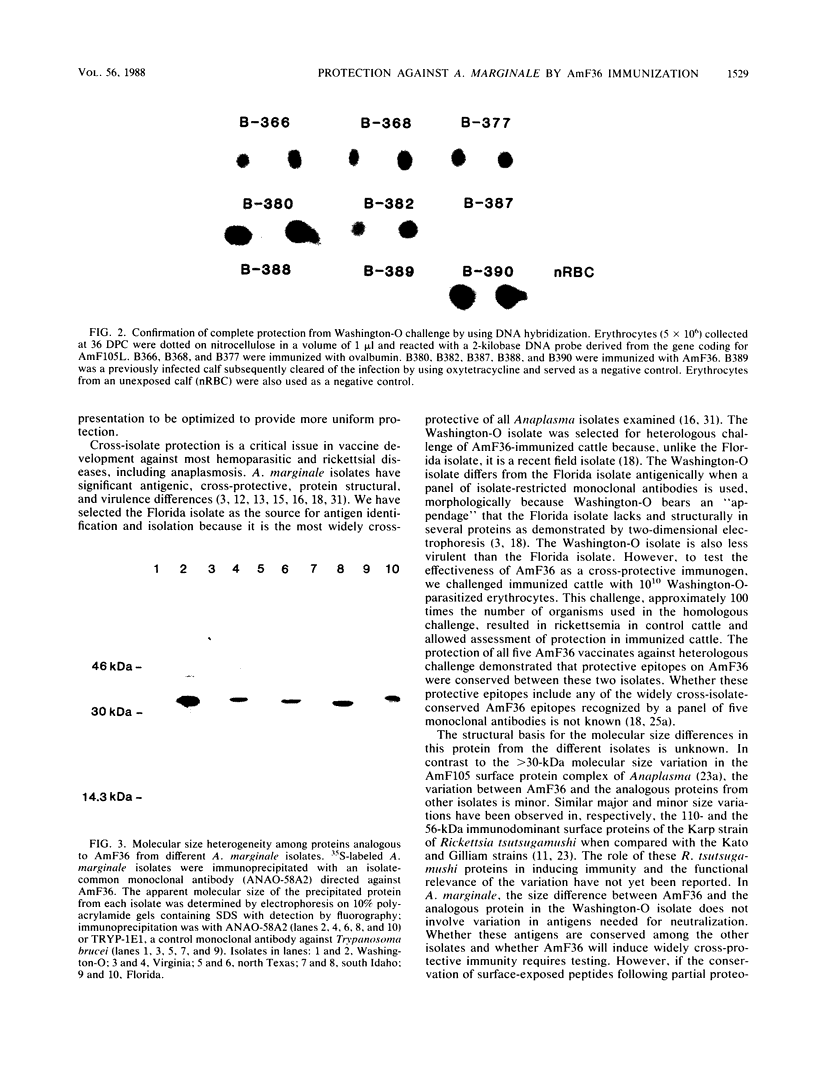
Immunization of cattle with a purified Anaplasma marginale major surface protein, AmF36, induced protection against homologous challenge with the Florida isolate. Similarly, immunized cattle were protected from challenge with the antigenically and structurally distinct Washington-O isolate of A. marginale. The degree of protection in AmF36-immunized cattle varied from complete prevention of rickettsemia to significant delay in the onset of rickettsemia compared with control immunized cattle. A single AmF36 vaccinate was not protected against homologous challenge despite development of a strong antibody response. Immunoprecipitation of A. marginale proteins with a monoclonal antibody to AmF36 identified minor molecular size heterogeneity in this protein from different isolates, including the Florida and Washington-O isolates. The apparent molecular size of this surface protein in the Florida isolate was 36 kilodaltons, whereas the analogous proteins in Washington-O and four other isolates of A. marginale from the United States had molecular masses of 33 to 34 kilodaltons. Significantly, the surface-exposed peptides of these proteins appear to be conserved among the different isolates. These results demonstrate the potential of AmF36 as a subunit immunogen for bovine anaplasmosis and indicate a structural basis for its cross-protective ability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985 Oct;135(4):2589–2592. [PubMed] [Google Scholar]
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck L., Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984 Aug;133(2):969–974. [PubMed] [Google Scholar]
- Carson C. A., Adams L. G., Todorovic R. A. An antigenic and serologic comparison of two virulent strains and an attenuated strain of Anaplasma marginale. Am J Vet Res. 1970 Jun;31(6):1071–1078. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Fandeur T., Dubois P., Gysin J., Dedet J. P., da Silva L. P. In vitro and in vivo studies on protective and inhibitory antibodies against Plasmodium falciparum in the Saimiri monkey. J Immunol. 1984 Jan;132(1):432–437. [PubMed] [Google Scholar]
- Hanson B. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect Immun. 1985 Dec;50(3):603–609. doi: 10.1128/iai.50.3.603-609.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. X. Morphologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:676–687. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. XI. Immunoserologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:688–696. [PubMed] [Google Scholar]
- Kuttler K. L., Winward L. D. Serologic comparisons of 4 Anaplasma isolates as measured by the complement-fixation test. Vet Microbiol. 1984 Apr;9(2):181–186. doi: 10.1016/0378-1135(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Kuttler K. L., Zaugg J. L., Johnson L. W. Serologic and clinical responses of premunized, vaccinated, and previously infected cattle to challenge exposure by two different Anaplasma marginale isolates. Am J Vet Res. 1984 Nov;45(11):2223–2226. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infections: protection against lethal Rickettsia tsutsugamushi infections by treatment of mice with macrophage-activating agents. J Leukoc Biol. 1984 Apr;35(4):385–396. doi: 10.1002/jlb.35.4.385. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Stover C. K., Rice R. M. Molecular cloning and expression of Rickettsia tsutsugamushi genes for two major protein antigens in Escherichia coli. Infect Immun. 1987 May;55(5):1156–1162. doi: 10.1128/iai.55.5.1156-1162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Kuttler K. L., McGuire T. C. Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol. 1986 Jun;23(6):1078–1083. doi: 10.1128/jcm.23.6.1078-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Kocan K. M., Barron S. J., Hair J. A., Barbet A. F., Davis W. C., McGuire T. C. Presence of common antigens, including major surface protein epitopes, between the cattle (intraerythrocytic) and tick stages of Anaplasma marginale. Infect Immun. 1985 Dec;50(3):881–886. doi: 10.1128/iai.50.3.881-886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Palmer G. H., Waghela S. D., Barbet A. F., Davis W. C., McGuire T. C. Characterization of a neutralization-sensitive epitope on the Am 105 surface protein of Anaplasma marginale. Int J Parasitol. 1987 Oct;17(7):1279–1285. doi: 10.1016/0020-7519(87)90093-2. [DOI] [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Wickwire K. B., Kocan K. M., Barron S. J., Ewing S. A., Smith R. D., Hair J. A. Infectivity of three Anaplasma marginale isolates for Dermacentor andersoni. Am J Vet Res. 1987 Jan;48(1):96–99. [PubMed] [Google Scholar]