Abstract
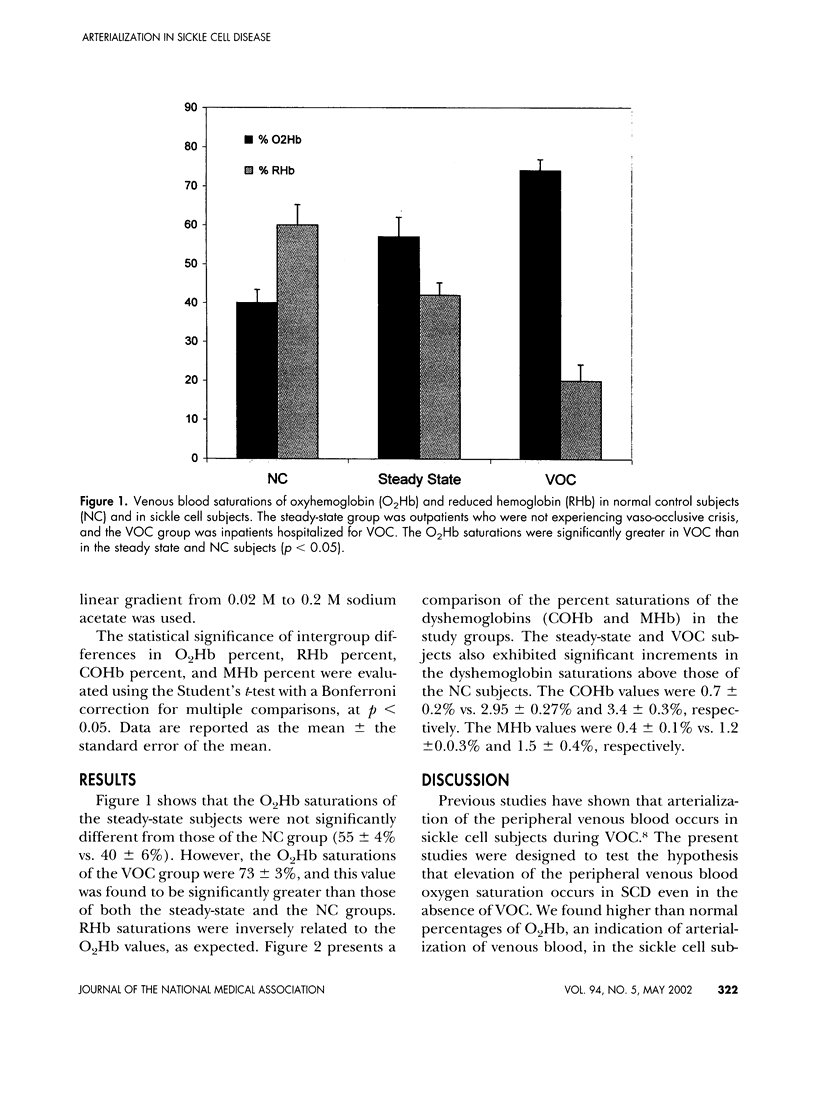
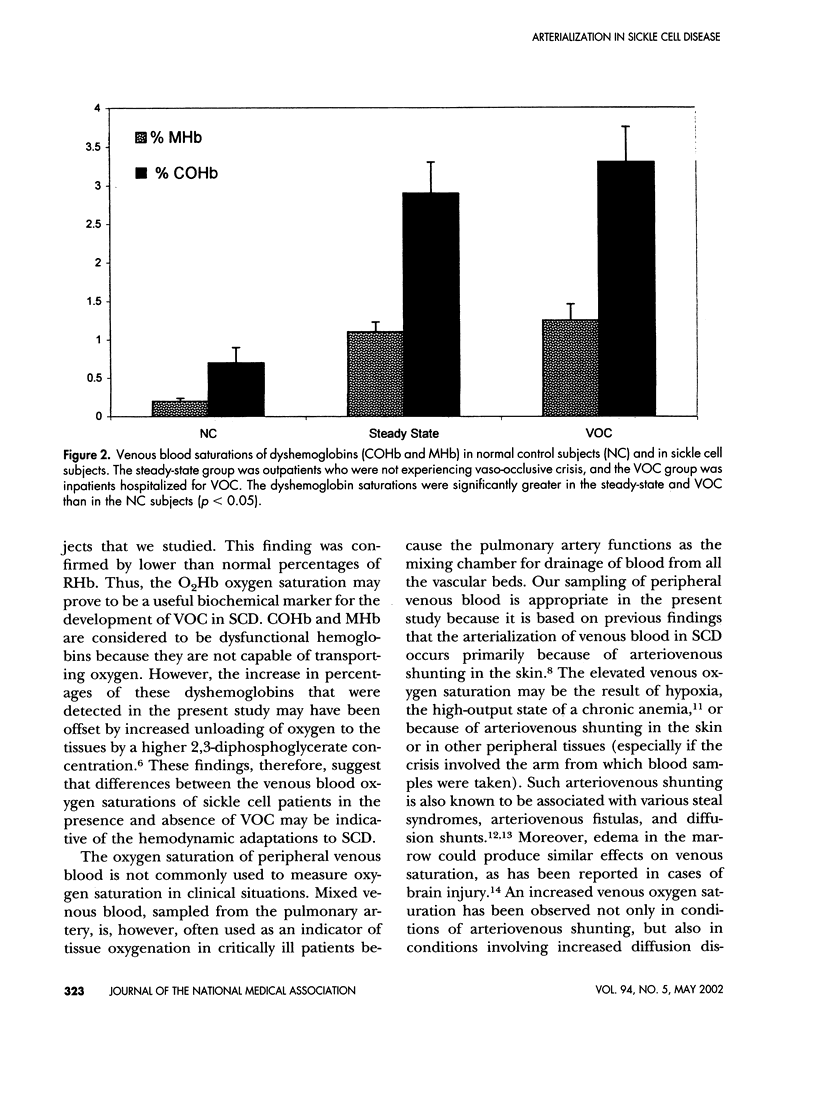
Arterialization of the venous blood is thought to be indicative of cutaneous shunting, and occurs in patients with sickle cell disease (SCD) during vaso-occlusive crisis (VOC). We performed the present study to quantify the amount of shunting that occurs in sickle cell patients presenting at the Howard University Sickle Cell Center, Washington, D.C., as outpatients and for hospitalizations associated with sickle cell crisis. Peripheral venous blood was drawn anaerobically into heparinized syringes from 9 normal control subjects (NC), 24 outpatients (steady-state group), and 14 inpatients during crisis (VOC group). Spectrophotometric measurements were made for the following species of hemoglobin (Hb): oxy-Hb (O2Hb), reduced Hb (RHb), carboxy-Hb (COHb), and met-Hb (MHb). In addition, fetal hemoglobin (HbF) was measured by high-pressure liquid chromatography (HPLC). The O2Hb saturations of the steady state group were not significantly different than those of the NC group (55 +/- 4% vs. 40 +/- 6%). However, the O2Hb saturations of the VOC group were 73 +/- 3%, and this value was found to be significantly greater than those of both the steady-state and the NC groups (p < 0.05). Reduced hemoglobin saturations were inversely related to the O2Hb values, as expected. Compared to the NC group, the steady-state, and VOC groups had greater dyshemoglobin (COHb and MHb) levels (p < 0.05). These findings suggest that the percentages of venous O2Hb and dyshemoglobins may be increased in sickle cell disease even in the absence of VOC. Therefore, the venous O2Hb saturation may be a useful biochemical marker for the arteriovenous shunting and hemodynamic adaptations associated with sickle cell disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleixandre A., López-Miranda V., Ortega A. Alpha-vascular responses after short-term and long-term inhibition of nitric oxide synthesis. J Cardiovasc Pharmacol. 2001 Feb;37(2):133–142. doi: 10.1097/00005344-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Castele R. J., Strohl K. P., Chester C. S., Brittenham G. M., Harris J. W. Oxygen saturation with sleep in patients with sickle cell disease. Arch Intern Med. 1986 Apr;146(4):722–725. [PubMed] [Google Scholar]
- Castro O., Osbaldiston G. W., Aponte L., Roth R., Orlin J., Finch S. C. Oxygen-dependent circulation of sickle erythrocytes. J Lab Clin Med. 1976 Nov;88(5):732–744. [PubMed] [Google Scholar]
- Chang J. Y., Liu L. Z. Catecholamines inhibit microglial nitric oxide production. Brain Res Bull. 2000 Aug;52(6):525–530. doi: 10.1016/s0361-9230(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Cheng C., Matsukawa T., Sessler D. I., Ozaki M., Kurz A., Merrifield B., Lin H., Olofsson P. Increasing mean skin temperature linearly reduces the core-temperature thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1995 May;82(5):1160–1168. doi: 10.1097/00000542-199505000-00011. [DOI] [PubMed] [Google Scholar]
- Cormio M., Valadka A. B., Robertson C. S. Elevated jugular venous oxygen saturation after severe head injury. J Neurosurg. 1999 Jan;90(1):9–15. doi: 10.3171/jns.1999.90.1.0009. [DOI] [PubMed] [Google Scholar]
- Curtis S. E., Walker T. A., Bradley W. E., Cain S. M. Raising P50 increases tissue PO2 in canine skeletal muscle but does not affect critical O2 extraction ratio. J Appl Physiol (1985) 1997 Nov;83(5):1681–1689. doi: 10.1152/jappl.1997.83.5.1681. [DOI] [PubMed] [Google Scholar]
- Goff C. D., Sato D. T., Bloch P. H., DeMasi R. J., Gregory R. T., Gayle R. G., Parent F. N., Meier G. H., Wheeler J. R. Steal syndrome complicating hemodialysis access procedures: can it be predicted? Ann Vasc Surg. 2000 Mar;14(2):138–144. doi: 10.1007/s100169910025. [DOI] [PubMed] [Google Scholar]
- Hofstra T. C., Kalra V. K., Meiselman H. J., Coates T. D. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood. 1996 May 15;87(10):4440–4447. [PubMed] [Google Scholar]
- Ikeda T., Ozaki M., Sessler D. I., Kazama T., Ikeda K., Sato S. Intraoperative phenylephrine infusion decreases the magnitude of redistribution hypothermia. Anesth Analg. 1999 Aug;89(2):462–465. doi: 10.1097/00000539-199908000-00040. [DOI] [PubMed] [Google Scholar]
- Lonsdorfer J., Bogui P., Otayeck A., Bursaux E., Poyart C., Cabannes R. Cardiorespiratory adjustments in chronic sickle cell anemia. Bull Eur Physiopathol Respir. 1983 Jul-Aug;19(4):339–344. [PubMed] [Google Scholar]
- Lutty G. A., Phelan A., McLeod D. S., Fabry M. E., Nagel R. L. A rat model for sickle cell-mediated vaso-occlusion in retina. Microvasc Res. 1996 Nov;52(3):270–280. doi: 10.1006/mvre.1996.0064. [DOI] [PubMed] [Google Scholar]
- MANFREDI F., SPOTO A. P., SALTZMAN H. A., SIEKER H. O. Studies of peripheral circulation during sickle-cell crisis. Circulation. 1960 Oct;22:602–607. doi: 10.1161/01.cir.22.4.602. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Weisman I. M., Zeballos R. J., Stephenson S. R. Exercise and hypoxia increase sickling in venous blood from an exercising limb in individuals with sickle cell trait. Am J Med. 1989 Jul;87(1):48–56. doi: 10.1016/s0002-9343(89)80482-6. [DOI] [PubMed] [Google Scholar]
- Osei S. Y., Ahima R. S., Fabry M. E., Nagel R. L., Bank N. Immunohistochemical localization of hepatic nitric oxide synthase in normal and transgenic sickle cell mice: the effect of hypoxia. Blood. 1996 Nov 1;88(9):3583–3588. [PubMed] [Google Scholar]
- Pakalnis V. A., Wolbarsht M. L., Landers M. B., 3rd Ocular oxygenation: the effect of phenylephrine on anterior chamber oxygen tension. Adv Exp Med Biol. 1986;200:233–241. doi: 10.1007/978-1-4684-5188-7_30. [DOI] [PubMed] [Google Scholar]
- SINGER K., SINGER L. Studies on abnormal hemoglobins. VIII. The gelling phenomenon of sickle cell hemoglobin: its biologic and diagnostic significance. Blood. 1953 Nov;8(11):1008–1023. [PubMed] [Google Scholar]
- Seakins M., Gibbs W. N., Milner P. F., Bertles J. F. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1973 Feb;52(2):422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Huang A., Recchia F. A., Cui Y., Messina E. J., Koller A., Kaley G. Nitric oxide-mediated arteriolar dilation after endothelial deformation. Am J Physiol Heart Circ Physiol. 2001 Feb;280(2):H714–H721. doi: 10.1152/ajpheart.2001.280.2.H714. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Bookchin R. M. Effects of carbon dioxide and pH variations in vitro on blood respiratory functions, red blood cell volume, transmembrane pH gradients, and sickling in sickle cell anemia. J Lab Clin Med. 1984 Aug;104(2):146–159. [PubMed] [Google Scholar]
- Wasserman K. Critical capillary PO2 and the role of lactate production in oxyhemoglobin dissociation during exercise. Adv Exp Med Biol. 1999;471:321–333. doi: 10.1007/978-1-4615-4717-4_39. [DOI] [PubMed] [Google Scholar]
- Wattanasirichaigoon S., Gordon F. D., Resnick R. H. Hyperdynamic circulation in portal hypertension: a comparative model of arterio-venous fistula. Med Hypotheses. 2000 Jul;55(1):77–87. doi: 10.1054/mehy.1999.1034. [DOI] [PubMed] [Google Scholar]
- Weinberg R. S., Acosta R., Knobloch M. E., Garber M., Alter B. P. Low oxygen enhances sickle and normal erythropoiesis and fetal hemoglobin synthesis in vitro. Hemoglobin. 1995 Sep;19(5):263–275. doi: 10.3109/03630269509005813. [DOI] [PubMed] [Google Scholar]
- Young R. C., Jr, Rachal R. E., Del Pilar Aguinaga M., Nelson B. L., Kim B. C., Winter W. P., Castro O. Automated oxyhemoglobin dissociation curve construction to assess sickle cell anemia therapy. J Natl Med Assoc. 2000 Sep;92(9):430–435. [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven M. A., Maertzdorf W. J., Blanco C. E. Relationship between mixed venous oxygen saturation and markers of tissue oxygenation in progressive hypoxic hypoxia and in isovolemic anemic hypoxia in 8- to 12-day-old piglets. Crit Care Med. 1999 Sep;27(9):1885–1892. doi: 10.1097/00003246-199909000-00029. [DOI] [PubMed] [Google Scholar]


