Abstract
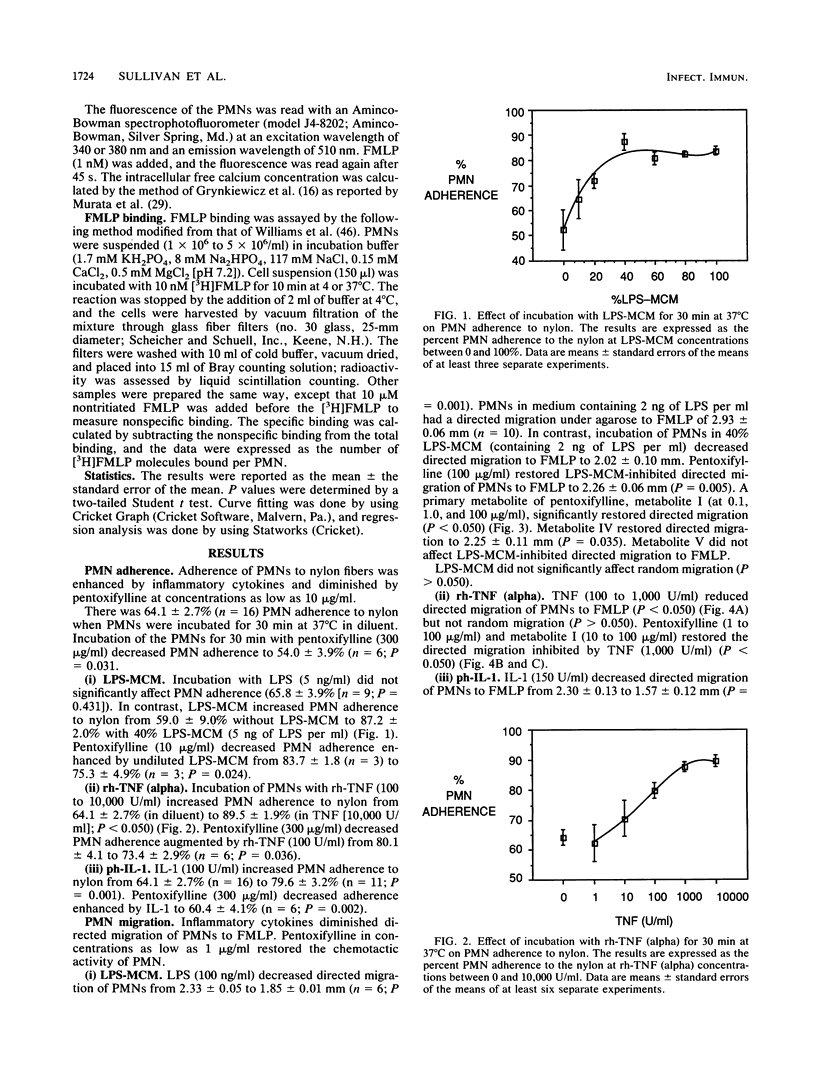
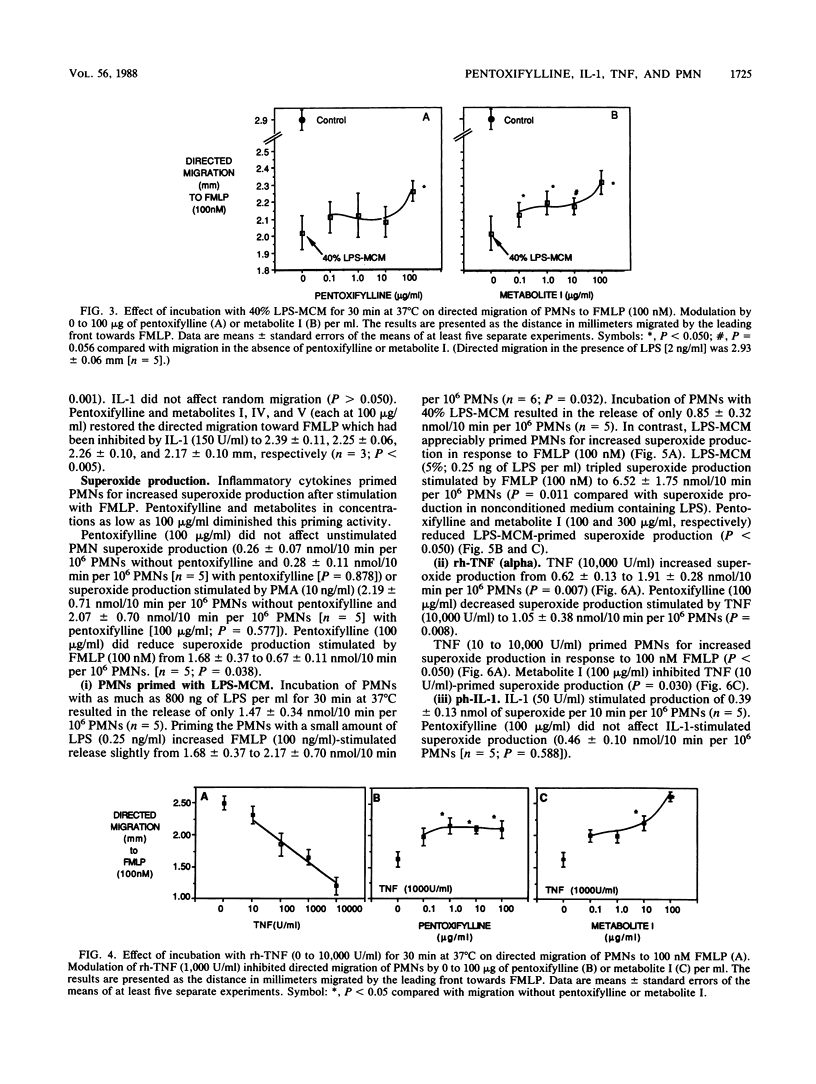
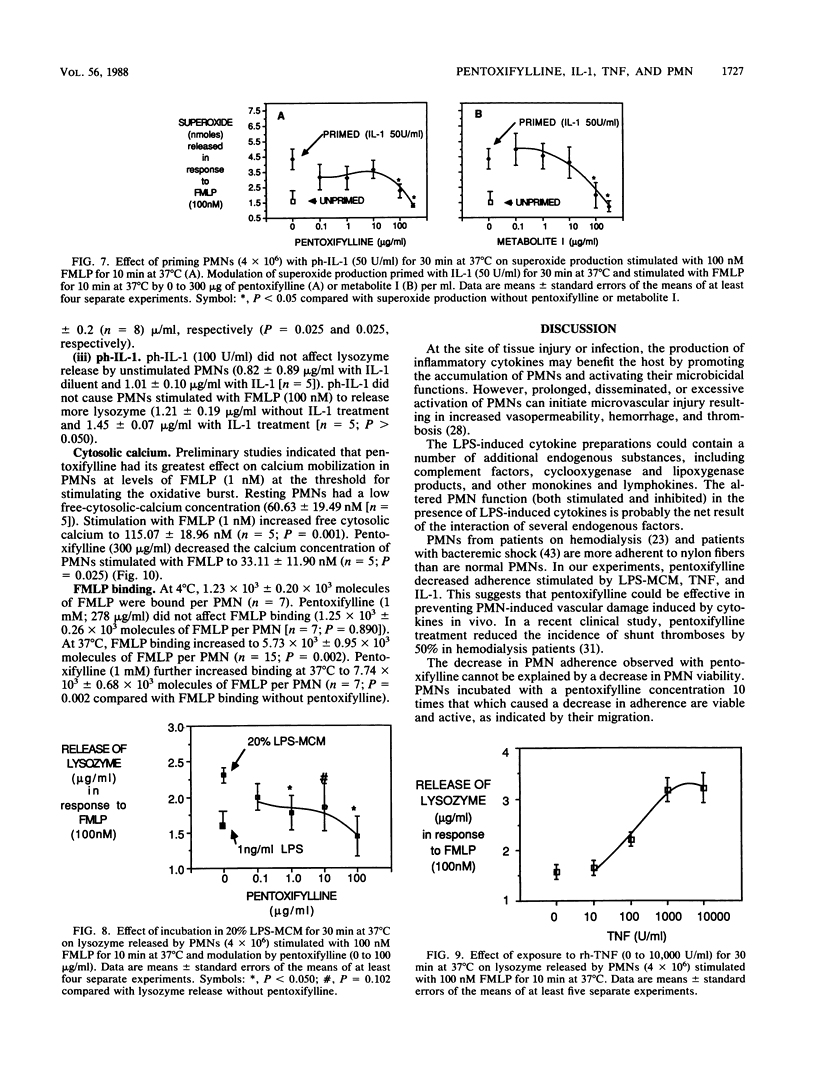
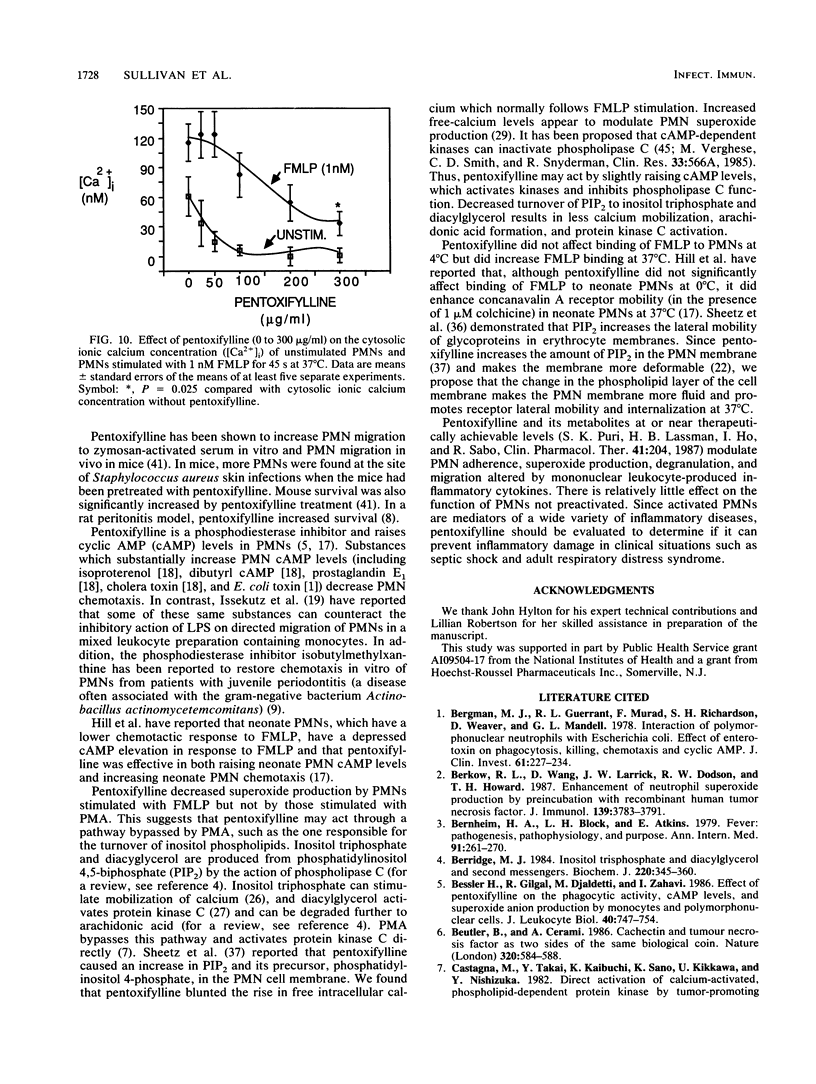
Inflammatory cytokines, including interleukin-1 and tumor necrosis factor, are produced by monocytes and macrophages in response to microorganisms and microbial products such as endotoxins. The cytokines stimulate neutrophil adherence, degranulation, and superoxide production but inhibit neutrophil migration. We studied the modulation of cytokine-induced neutrophil activation by pentoxifylline and its principle metabolites. Lipopolysaccharide-stimulated mononuclear-leukocyte-conditioned medium containing inflammatory cytokines, purified human interleukin-1, or recombinant human tumor necrosis factor increased neutrophil adherence to nylon fiber, primed neutrophils for increased superoxide production in response to N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP), increased neutrophil lysozyme release stimulated by FMLP, and decreased directed migration of neutrophils to FMLP. Pentoxifylline and its principle metabolites at or near therapeutically achievable levels were able to counteract these effects. Pentoxifylline inhibited the increase in free intracellular calcium in polymorphonuclear leukocytes stimulated by FMLP and increased binding of FMLP to neutrophils at 37 degrees C but not at 4 degrees C. By blocking the inflammatory action of interleukin-1 and tumor necrosis factor on neutrophils, pentoxifylline may diminish the tissue damage caused by neutrophils in such conditions as septic shock, adult respiratory distress syndrome, cardiopulmonary bypass lung damage, and myocardial reperfusion injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman M. J., Guerrant R. L., Murad F., Richardson S. H., Weaver D., Mandell G. L. Interaction of polymorphonuclear neutrophils with Escherichia coli. Effect of enterotoxin on phagocytosis, killing, chemotaxis, and cyclic AMP. J Clin Invest. 1978 Feb;61(2):227–234. doi: 10.1172/JCI108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Bernheim H. A., Block L. H., Atkins E. Fever: pathogenesis, pathophysiology, and purpose. Ann Intern Med. 1979 Aug;91(2):261–270. doi: 10.7326/0003-4819-91-2-261. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler H., Gilgal R., Djaldetti M., Zahavi I. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J Leukoc Biol. 1986 Dec;40(6):747–754. doi: 10.1002/jlb.40.6.747. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chalkiadakis G. E., Kostakis A., Karayannacos P. E., Chalkiadakis M. E., Sgouromali S., Giamarellou H., Skalkeas G. D. Pentoxifylline in the treatment of experimental peritonitis in rats. Arch Surg. 1985 Oct;120(10):1141–1144. doi: 10.1001/archsurg.1985.01390340039007. [DOI] [PubMed] [Google Scholar]
- Debski B. F., Ranney R. R., Carchman R. A. The alterative effect of isobutylmethylxanthine in hypofunctional human neutrophil chemotaxis. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1228–1234. doi: 10.1016/0006-291x(82)92131-3. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dooley D. C., Simpson J. F., Meryman H. T. Isolation of large numbers of fully viable human neutrophils: a preparative technique using percoll density gradient centrifugation. Exp Hematol. 1982 Aug;10(7):591–599. [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hill H. R., Augustine N. H., Newton J. A., Shigeoka A. O., Morris E., Sacchi F. Correction of a developmental defect in neutrophil activation and movement. Am J Pathol. 1987 Aug;128(2):307–314. [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Estensen R. D., Quie P. G., Hogan N. A., Goldberg N. D. Modulation of human neutrophil chemotactic responses by cyclic 3',5'-guanosine monophosphate and cyclic 3',5'-adenosine monophosphate. Metabolism. 1975 Mar;24(3):447–456. doi: 10.1016/0026-0495(75)90124-9. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Ng M., Biggar W. D. Effect of cyclic adenosine 3',5'-monophosphate antagonists on endotoxin-induced inhibition of human neutrophil chemotaxis. Infect Immun. 1979 May;24(2):434–440. doi: 10.1128/iai.24.2.434-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Gallin J. I. Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest. 1978 May;61(5):1330–1336. doi: 10.1172/JCI109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Gregor R. R. Granulocyte adherence changes induced by hemodialysis, endotoxin, epinephrine, and glucocorticoids. Ann Intern Med. 1977 Jan;86(1):35–39. doi: 10.7326/0003-4819-86-1-35. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Raised serum levels of cachectin/tumor necrosis factor alpha in renal allograft rejection. J Exp Med. 1987 Oct 1;166(4):1132–1137. doi: 10.1084/jem.166.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Niedel J. E., Cambier J. C. B cell activation. IV. Induction of cell membrane depolarization and hyper-I-A expression by phorbol diesters suggests a role for protein kinase C in murine B lymphocyte activation. J Immunol. 1984 Mar;132(3):1472–1478. [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I., Colditz I. G., Chan M. K., Dinarello C. A. Acute inflammation in gram-negative infection: endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed Proc. 1987 Jan;46(1):97–104. [PubMed] [Google Scholar]
- Murata T., Sullivan J. A., Sawyer D. W., Mandell G. L. Influence of type and opsonization of ingested particle on intracellular free calcium distribution and superoxide production by human neutrophils. Infect Immun. 1987 Aug;55(8):1784–1791. doi: 10.1128/iai.55.8.1784-1791.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Radmilović A., Borić Z., Naumović T., Stamenković M., Muśikić P. Shunt thrombosis prevention in hemodialysis patients--a double-blind, randomized study: pentoxifylline vs placebo. Angiology. 1987 Jul;38(7):499–506. doi: 10.1177/000331978703800701. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Shalaby M. R., Palladino M. A., Jr, Hirabayashi S. E., Eessalu T. E., Lewis G. D., Shepard H. M., Aggarwal B. B. Receptor binding and activation of polymorphonuclear neutrophils by tumor necrosis factor-alpha. J Leukoc Biol. 1987 Mar;41(3):196–204. doi: 10.1002/jlb.41.3.196. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Febbroriello P., Koppel D. E. Triphosphoinositide increases glycoprotein lateral mobility in erythrocyte membranes. Nature. 1982 Mar 4;296(5852):91–93. doi: 10.1038/296091a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Wang W. P., Kreutzer D. L. Polyphosphoinositides as regulators of membrane skeletal stability. Kroc Found Ser. 1984;16:87–94. [PubMed] [Google Scholar]
- Smith R. J., Speziale S. C., Bowman B. J. Properties of interleukin-1 as a complete secretagogue for human neutrophils. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1233–1240. doi: 10.1016/0006-291x(85)91746-2. [DOI] [PubMed] [Google Scholar]
- Solomons N. W., Elson C. O., Pekarek R. S., Jacob R. A., Sandstead H. H., Rosenberg I. H. Leukocytic endogenous mediator in Crohn's disease. Infect Immun. 1978 Dec;22(3):637–639. doi: 10.1128/iai.22.3.637-639.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Patselas T. N., Redick J. A., Mandell G. L. Enhancement of chemotaxis and protection of mice from infection. Trans Assoc Am Physicians. 1984;97:337–345. [PubMed] [Google Scholar]
- Venezio F. R., Westenfelder G. O., Phair J. P. The adherence of polymorphonuclear leukocytes in patients with sepsis. J Infect Dis. 1982 Mar;145(3):351–357. doi: 10.1093/infdis/145.3.351. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


