Abstract
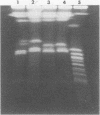
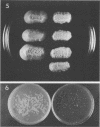
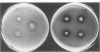
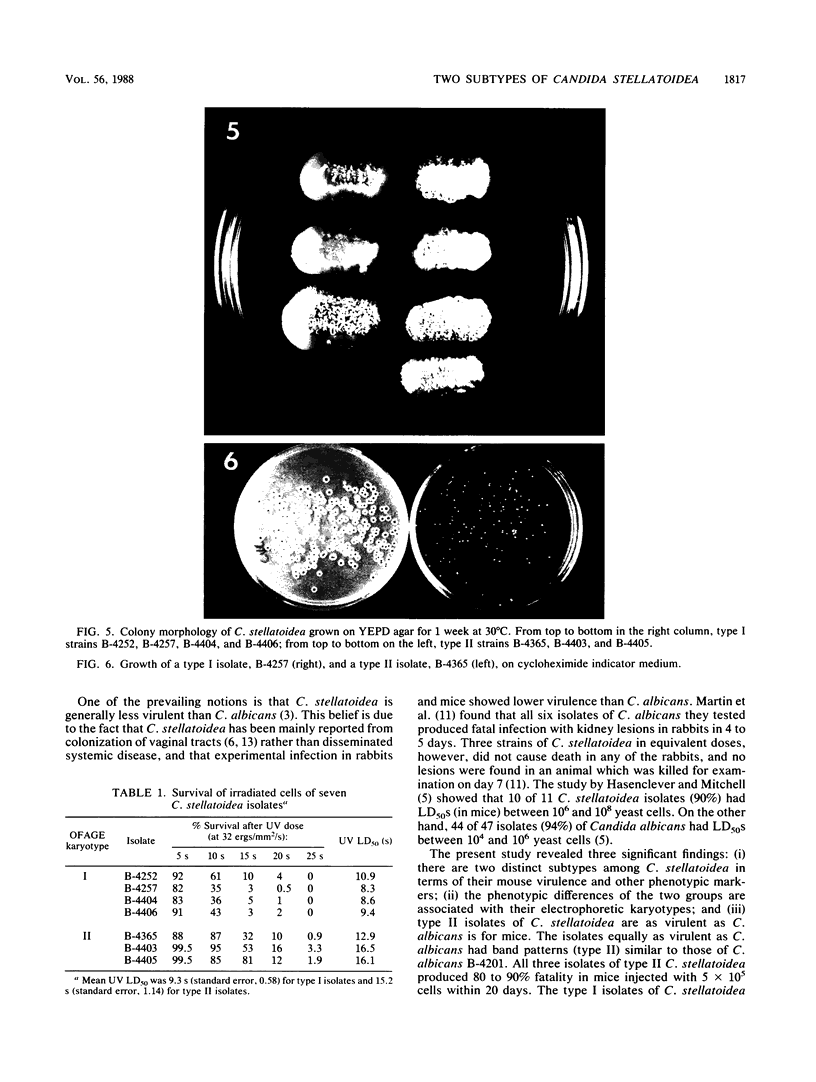
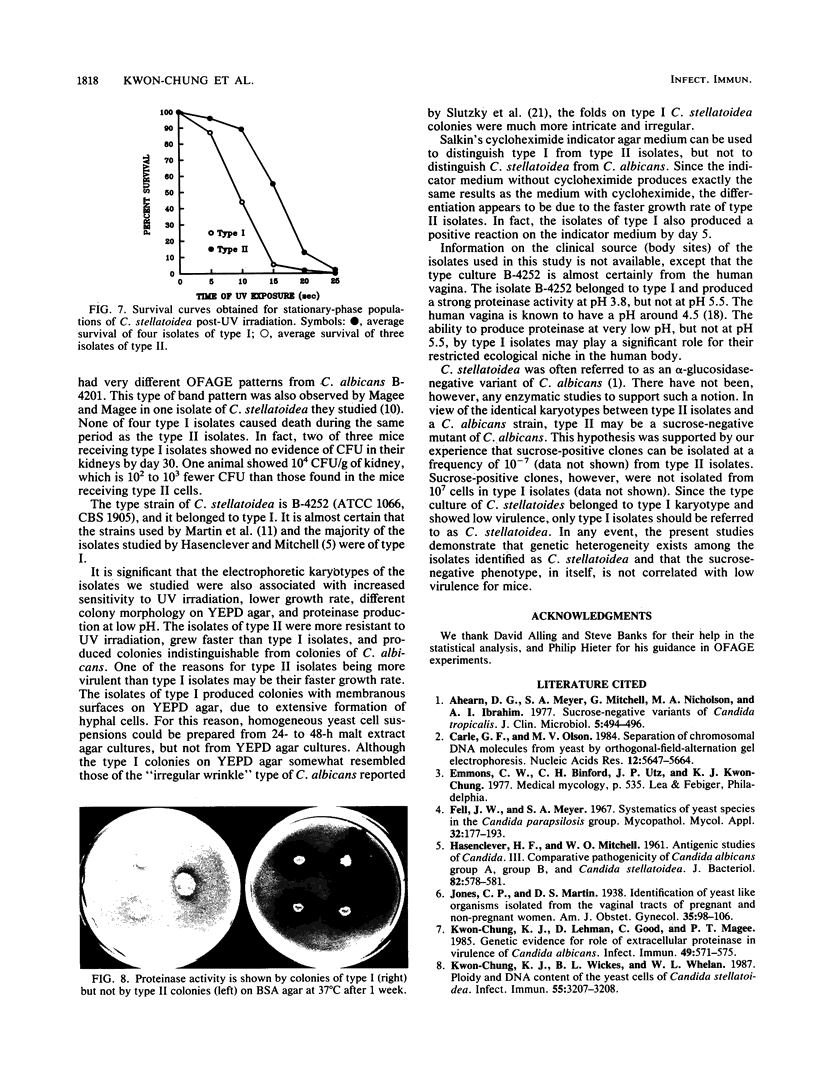
Seven isolates of Candida stellatoidea were studied for their electrophoretic karyotype, virulence for mice, sensitivity to UV radiation, growth rate in vitro, reaction on cycloheximide-indicator medium, and proteinase activity. The isolates exhibited one of two distinct electrophoretic karyotypes as determined by orthogonal field alternating gel electrophoresis (OFAGE). Four isolates, including the type culture of C. stellatoidea, belonged to electrophoretic karyotype type I by OFAGE, showing eight to nine bands of which at least two bands were less than 1,000 kilobases in size as estimated by comparison with the DNA bands of Saccharomyces cerevisiae. These isolates failed to produce fatal infection in mice within 20 days when 5 X 10(5) cells were injected intravenously. The yeasts were cleared from the kidneys of two of three mice tested by day 30. Type I showed proteinase activity on bovine serum albumin agar at pH 3.8 and produced a negative reaction on cycloheximide-bromcresol green medium within 48 h. The three grouped in type II by OFAGE showed banding patterns similar to those of a well-characterized isolate of Candida albicans. The isolates of type II had an electrophoretic karyotype of six to seven bands approximately 1,200 kilobases or greater in size. All three type II isolates were highly virulent for mice, producing fatality curves similar to those of a previously studied C. albicans isolate. From 80 to 90% of the mice injected with 5 X 10(5) cells intravenously died within 20 days. The type II isolates produced a positive reaction on cycloheximide-bromcresol green agar and showed no proteinase activity on bovine serum albumin agar at the low pH. In addition, the type II isolates grew faster and were significantly more resistant to UV irradiation than the type I isolates. These results indicated that type II, but not type I, isolates can be considered simply as sucrose-negative C. albicans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G., Meyer S. A., Mitchell G., Nicholson M. A., Ibrahim A. I. Sucrose-negative variants of Candida tropicalis. J Clin Microbiol. 1977 Apr;5(4):494–496. doi: 10.1128/jcm.5.4.494-496.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J. W., Meyer S. A. Systematics of yeast species in the Candida parapsilosis group. Mycopathol Mycol Appl. 1967 Aug 14;32(3):177–193. doi: 10.1007/BF02049795. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Antigenic studies of Candida. III. Comparative pathogenicity of Candida albicans group A, group B, and Candida stellatoidea. J Bacteriol. 1961 Oct;82:578–581. doi: 10.1128/jb.82.4.578-581.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Wickes B. L., Whelan W. L. Ploidy and DNA content of Candida stellatoidea cells. Infect Immun. 1987 Dec;55(12):3207–3208. doi: 10.1128/iai.55.12.3207-3208.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., D'Souza T. M., Magee P. T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987 Apr;169(4):1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Magee P. T. Electrophoretic karyotypes and chromosome numbers in Candida species. J Gen Microbiol. 1987 Feb;133(2):425–430. doi: 10.1099/00221287-133-2-425. [DOI] [PubMed] [Google Scholar]
- Martin D. S., Jones C. P., Yao K. F., Lee L. E. A Practical Classification of the Monilias. J Bacteriol. 1937 Jul;34(1):99–129. doi: 10.1128/jb.34.1.99-129.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. M., Lasker B. A., Riggsby W. S. Molecular probe for identification of medically important Candida species and Torulopsis glabrata. J Clin Microbiol. 1987 Mar;25(3):563–566. doi: 10.1128/jcm.25.3.563-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- Ryley J. F. Pathogenicity of Candida albicans with particular reference to the vagina. J Med Vet Mycol. 1986 Feb;24(1):5–22. [PubMed] [Google Scholar]
- Salkin I. F. New medium for differentiation of Candida albicans from Candida stellatoidea. J Clin Microbiol. 1979 Apr;9(4):551–553. doi: 10.1128/jcm.9.4.551-553.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Snell R. G., Wilkins R. J. Separation of chromosomal DNA molecules from C.albicans by pulsed field gel electrophoresis. Nucleic Acids Res. 1986 Jun 11;14(11):4401–4406. doi: 10.1093/nar/14.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]