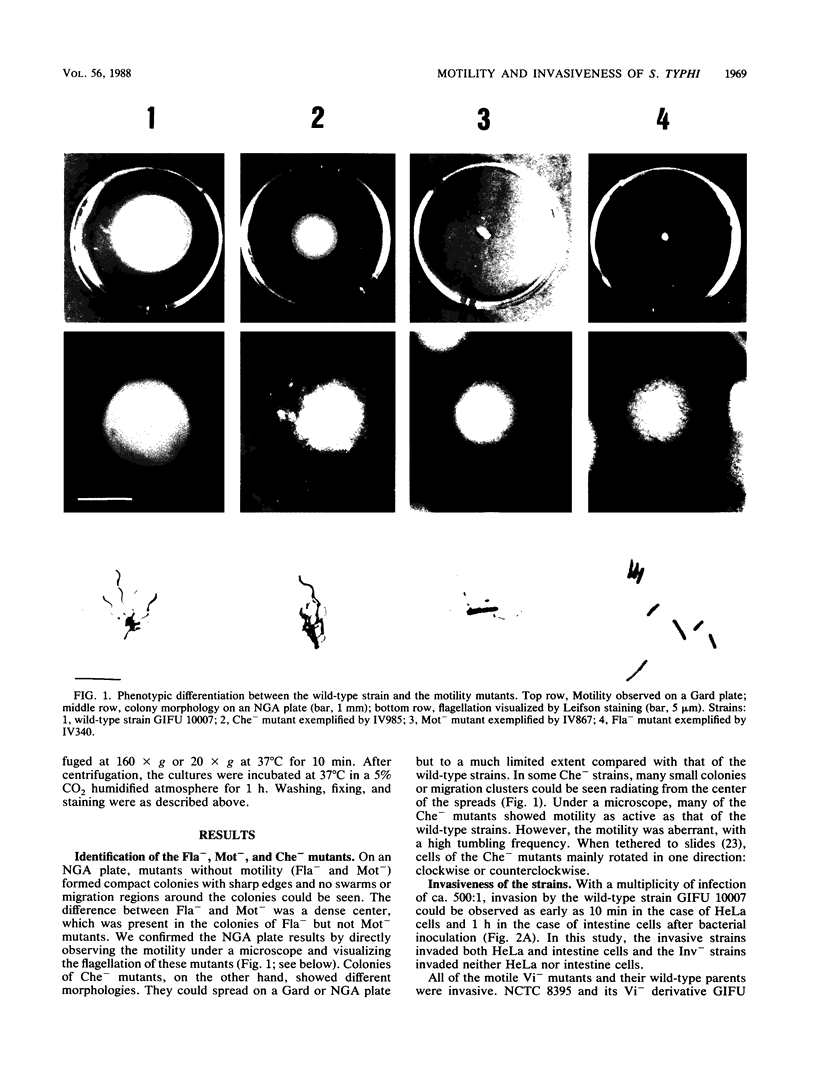
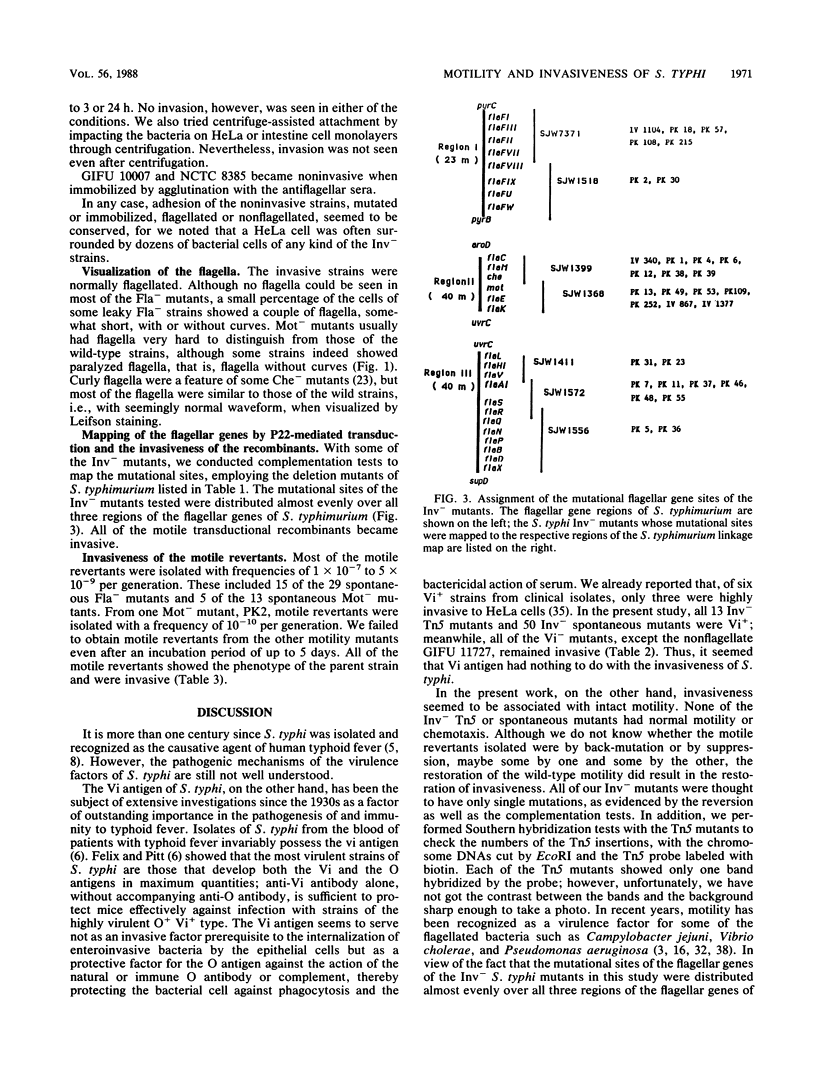
Abstract
Invasiveness of Salmonella typhi was investigated. At first, we introduced Tn5 into the chromosome of a wild-type S. typhi strain, GIFU 10007, and screened the independent Tn5 insertion mutants for noninvasive (Inv-) strains. During the first half of this work, we obtained 4 Inv- strains from 1,338 independent Tn5 mutants. The four were either nonflagellate (Fla-), nonmotile (Mot-), or nonchemotactic (Che-). We then isolated more Fla-, Mot-, or Che- mutants and examined the invasiveness of these mutants. Sixty-three spontaneous or Tn5 insertion motility mutants, i.e., Fla-, Mot-, or Che-, were independently isolated from the wild-type strain GIFU 10007; all of them were noninvasive. Motile revertants isolated from some of these mutants showed the same invasiveness as the parent strain. P22-mediated transductional crosses were carried out between some of the motility mutants (as the recipients) and the Fla- reference strains of S. typhimurium with known deletion sites on the genome (as the donors). The mutational sites of the S. typhi mutants were assigned almost evenly to the three flagellar gene regions (regions I, II, and III) of S. typhimurium. The invasiveness of the motile recombinants obtained from the transduction assays was examined. The restoration of intact motility resulted in the restoration of invasiveness. Thus, we conclude that intact motility is an invasion-related factor of S. typhi. The relationship of Vi antigen to the invasiveness of S. typhi was also studied. Vi-negative mutants with intact motility remained invasive, whereas all 63 Inv- spontaneous or Tn5 mutants were Vi positive. Therefore, Vi antigen was not related to the invasiveness of S. typhi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allweiss B., Dostal J., Carey K. E., Edwards T. F., Freter R. The role of chemotaxis in the ecology of bacterial pathogens of mucosal surfaces. Nature. 1977 Mar 31;266(5601):448–450. doi: 10.1038/266448a0. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Lushbaugh W. B., Inman L. R. Attachment of bacteria to intestinal epithelial cells in diarrhea caused by Escherichia coli strain RDEC-1 in the rabbit: stages and role of capsule. J Infect Dis. 1981 Feb;143(2):219–230. doi: 10.1093/infdis/143.2.219. [DOI] [PubMed] [Google Scholar]
- Craven R. C., Montie T. C. Motility and chemotaxis of three strains of Pseudomonas aeruginosa used for virulence studies. Can J Microbiol. 1981 Apr;27(4):458–460. doi: 10.1139/m81-070. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Formal S. B., Hornick R. B., Snyder M. J., Libonati J. P., Sheahan D. G., LaBrec E. H., Kalas J. P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971 Jul 1;285(1):1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- Freter R. Mechanisms of association of bacteria with mucosal surfaces. Ciba Found Symp. 1981;80:36–55. doi: 10.1002/9780470720639.ch4. [DOI] [PubMed] [Google Scholar]
- GERBER D. F., WATKINS H. M. Growth of shigellae in monolayer tissue cultures. J Bacteriol. 1961 Dec;82:815–822. doi: 10.1128/jb.82.6.815-822.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Richardson L. A., Uhlman D. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J Gen Microbiol. 1981 Dec;127(2):351–360. doi: 10.1099/00221287-127-2-351. [DOI] [PubMed] [Google Scholar]
- Kihlström E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977 Aug;17(2):290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacterial virulence factors--with particular reference to Salmonella bacteria. Scand J Infect Dis Suppl. 1980;Suppl 24:86–92. [PubMed] [Google Scholar]
- Lindquist B. L., Lebenthal E., Lee P. C., Stinson M. W., Merrick J. M. Adherence of Salmonella typhimurium to small-intestinal enterocytes of the rat. Infect Immun. 1987 Dec;55(12):3044–3050. doi: 10.1128/iai.55.12.3044-3050.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- Mehlman I. J., Eide E. L., Sanders A. C., Fishbein M., Aulisio C. C. Methodology for recognition of invasive potential of Escherichia coli. J Assoc Off Anal Chem. 1977 May;60(3):546–562. [PubMed] [Google Scholar]
- Ogawa H., Nakamura A., Sakazaki R. Pathogenic properties of "enteropathogenic" Escherichia coli from diarrheal children and adults. Jpn J Med Sci Biol. 1968 Oct;21(5):333–349. [PubMed] [Google Scholar]
- Okamura N., Nagai T., Nakaya R., Kondo S., Murakami M., Hisatsune K. HeLa cell invasiveness and O antigen of Shigella flexneri as separate and prerequisite attributes of virulence to evoke keratoconjunctivitis in guinea pigs. Infect Immun. 1983 Feb;39(2):505–513. doi: 10.1128/iai.39.2.505-513.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. A series of Tn5 variants with various drug-resistance markers and suicide vector for transposon mutagenesis. Gene. 1987;56(2-3):283–288. doi: 10.1016/0378-1119(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Ueki Y., Umeda A., Fujimoto S., Mitsuyama M., Amako K. Protection against Campylobacter jejuni infection in suckling mice by anti-flagellar antibody. Microbiol Immunol. 1987;31(12):1161–1171. doi: 10.1111/j.1348-0421.1987.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Uhlman D. L., Jones G. W. Chemotaxis as a factor in interactions between HeLa cells and Salmonella typhimurium. J Gen Microbiol. 1982 Feb;128(2):415–418. doi: 10.1099/00221287-128-2-415. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Nurmi T., Mäki M., Skurnik M., Sundqvist C., Granfors K., Grönroos P. Plasmids in Yersinia enterocolitica serotypes O:3 and O:9: correlation with epithelial cell adherence in vitro. Infect Immun. 1981 Sep;33(3):870–876. doi: 10.1128/iai.33.3.870-876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi E., Ikedo M., Ezaki T. Invasiveness of Salmonella typhi strains in HeLa S3 monolayer cells. Microbiol Immunol. 1986;30(12):1213–1224. doi: 10.1111/j.1348-0421.1986.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Fujita H., Ishihara A., Aizawa S., Macnab R. M. Subdivision of flagellar genes of Salmonella typhimurium into regions responsible for assembly, rotation, and switching. J Bacteriol. 1986 Apr;166(1):187–193. doi: 10.1128/jb.166.1.187-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Fujita H., Taira T., Kutsukake K., Homma M., Iino T. Genetic analysis of three additional fla genes in Salmonella typhimurium. J Gen Microbiol. 1984 Dec;130(12):3339–3342. doi: 10.1099/00221287-130-12-3339. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Flagella-induced immunity against experimental cholera in adult rabbits. Infect Immun. 1979 Jul;25(1):220–228. doi: 10.1128/iai.25.1.220-228.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]