Abstract
To investigate the potential pathogenic mechanisms of the oral periodontopathogen Wolinella recta ATCC 33238, we have isolated its lipopolysaccharide (LPS) and determined the chemical composition and selected in vitro biological activities of the molecule. Sodium desoxycholate-polyacrylamide gel electrophoresis revealed the W. recta LPS to be an atypical smooth LPS with short O-antigenic side chains. Chemically the LPS consisted of 47.2% lipid A, 19.6% polysaccharide, 9.0% heptose, 8.5% hexosamine, 3.2% phosphate, and 0.6% 2-keto-3-deoxyoctanoate. The major fatty acids were hexadecanoic acid (25.0%), 3-OH tetradecanoic acid (23.8%), tetradecanoic acid (15.4%), 3-OH hexadecanoic acid (11.6%), and octadecenoic acid (10.9%). Rhamnose constituted 87.8% of the carbohydrates generally associated with the O antigen, with smaller amounts of glucose (5.5%), mannose (4.9%), and an unidentified sugar (1.9%). CD-1 and C3H/HeN macrophages (M phi) exposed to 1 microgram of W. recta LPS per ml released 6.0 and 10.5 ng of prostaglandin E per ml of supernatant, representing 625% and 1,306% of prostaglandin E release by the control (without LPS). Maximum prostaglandin E release occurred in CD-1 M phi exposed to 100 micrograms of LPS per ml and was equivalent to 1,542% of release by the control. Interleukin-1 (IL-1) activities in CD-1 and C3H/HeN M phi exposed to 1 micrograms of LPS per ml were 257% and 1,941% of activities in the control, respectively. Maximum IL-1 release in CD-1 M phi occurred in response to 50 micrograms of LPS per ml and represented a 927% increase over release in the control, while 100 micrograms LPS per ml stimulated maximum IL-1 release in C3H/HeN M phi that was greater than 5,000% of release by the control.
Full text
PDF

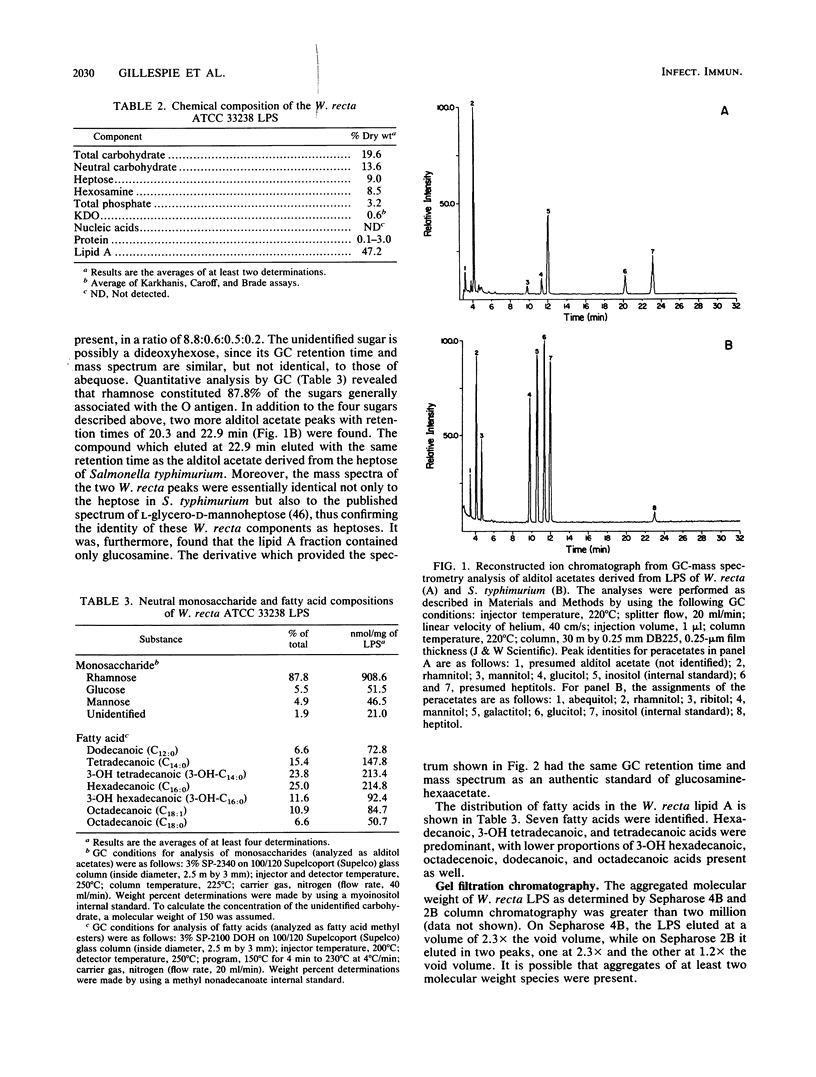
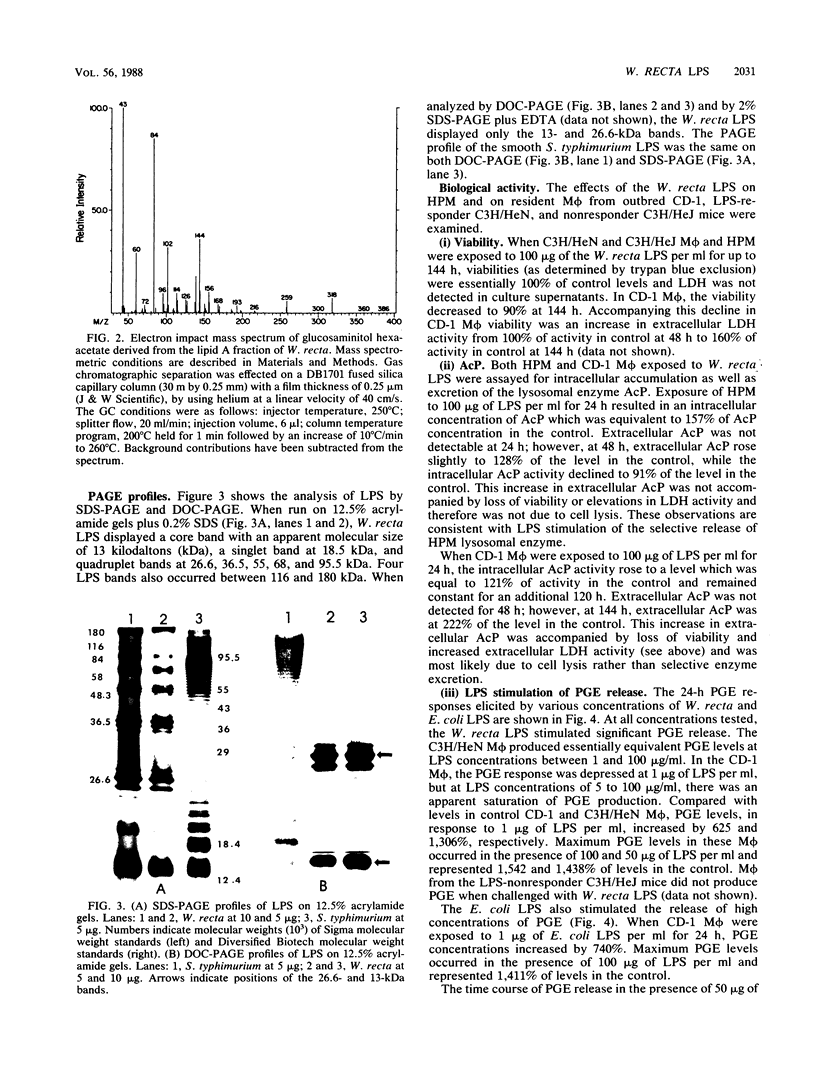
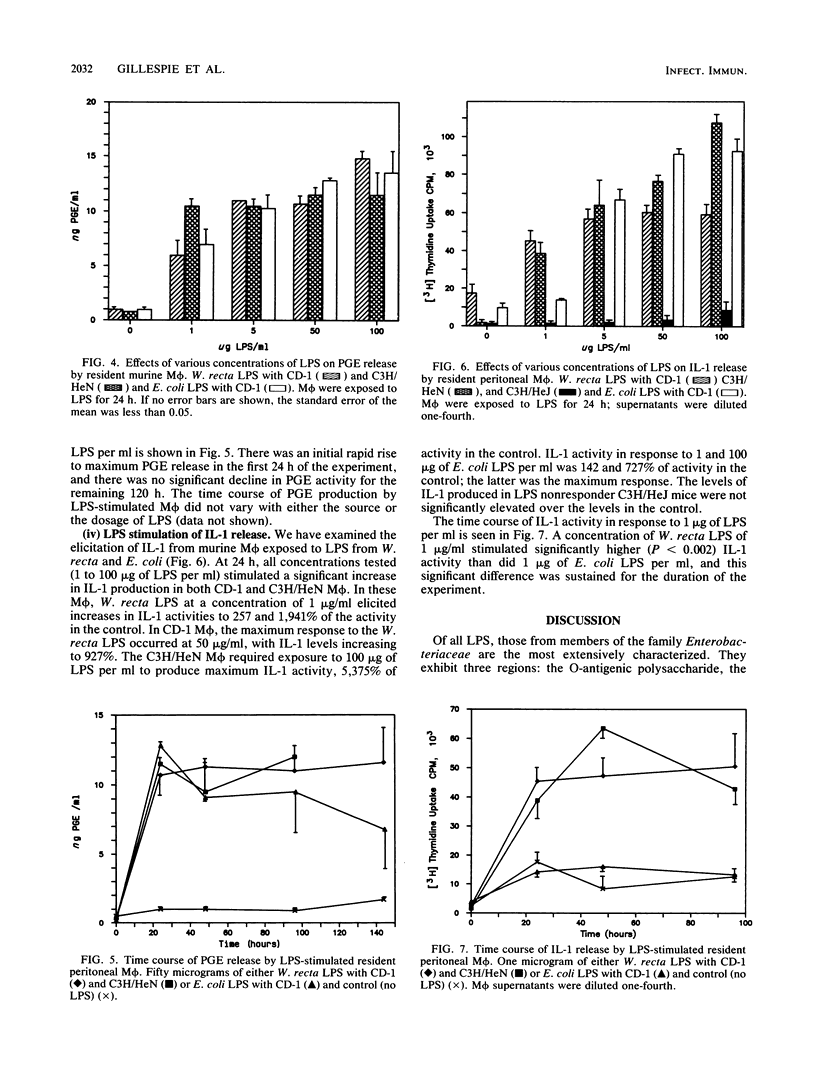



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Augustyniak H., Augustyniak J. Anhydride-type basic artifacts of amino acids. Anal Biochem. 1966 Apr;15(1):163–167. doi: 10.1016/0003-2697(66)90261-2. [DOI] [PubMed] [Google Scholar]
- Barker K. A., Holt S. C. The in vitro effect of Actinobacillus actinomycetemcomitans strain Y4 lipopolysaccharide on murine peritoneal macrophages. Can J Microbiol. 1983 Nov;29(11):1552–1563. doi: 10.1139/m83-238. [DOI] [PubMed] [Google Scholar]
- Basu S., Radziejewska-Lebrecht J., Mayer H. Lipopolysaccharide of Providencia rettgeri. Chemical studies and taxonomical implications. Arch Microbiol. 1986 Apr;144(3):213–218. doi: 10.1007/BF00410949. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- CABAUD P. G., WROBLEWSKI F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol. 1958 Sep;30(3):234–236. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Galanos C., Hansen-Hagge T., Lehmann V., Lüderitz O. Comparison of the capacity of two lipid A precursor molecules to express the local Shwartzman phenomenon. Infect Immun. 1985 May;48(2):355–358. doi: 10.1128/iai.48.2.355-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J., Holt S. C. Growth studies of Wolinella recta, a gram-negative periodontopathogen. Oral Microbiol Immunol. 1987 Sep;2(3):105–111. doi: 10.1111/j.1399-302x.1987.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Haapasalo M. Bacteroides buccae and related taxa in necrotic root canal infections. J Clin Microbiol. 1986 Dec;24(6):940–944. doi: 10.1128/jcm.24.6.940-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Nakada K., Ohmori Y., Miyoshi T., Amano S., Kitano S. Functional role of interleukin 1 in periodontal disease: induction of interleukin 1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect Immun. 1985 Oct;50(1):262–270. doi: 10.1128/iai.50.1.262-270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. K., Atkinson S. J., Hembry R. M., Reynolds J. J., Meikle M. C. Bacterial antigens induce collagenase and prostaglandin E2 synthesis in human gingival fibroblasts through a primary effect on circulating mononuclear cells. Infect Immun. 1987 Sep;55(9):2148–2154. doi: 10.1128/iai.55.9.2148-2154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. R., McKinney K. L., Marks M. I., Hyde R. M. Comparative analysis of Haemophilus influenzae type b and Escherichia coli J5 lipopolysaccharides. J Med Microbiol. 1986 Feb;21(1):25–33. doi: 10.1099/00222615-21-1-25. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johne B., Bryn K. Chemical composition and biological properties of a lipopolysaccharide from Bacteroides intermedius. Acta Pathol Microbiol Immunol Scand B. 1986 Aug;94(4):265–271. doi: 10.1111/j.1699-0463.1986.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kiley P., Holt S. C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980 Dec;30(3):862–873. doi: 10.1128/iai.30.3.862-873.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Nishihara T., Fujiwara T., Nisizawa T., Okahashi N., Noguchi T., Hamada S. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985 Mar;47(3):638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Takahashi I., Tsujimoto M., Ogawa T., Ikeda T., Harada K., Okamura H., Tamura T., Tanaka S. Low endotoxic activities of synthetic Salmonella-type lipid A with an additional acyloxyacyl group on the 2-amino group of beta (1-6) glucosamine disaccharide 1,4'-bisphosphate. Infect Immun. 1986 Jun;52(3):872–884. doi: 10.1128/iai.52.3.872-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löning T., Albers H. K., Lisboa B. P., Burkhardt A., Caselitz J. Prostaglandin E and the local immune response in chronic periodontal disease. Immunohistochemical and radioimmunological observations. J Periodontal Res. 1980 Sep;15(5):525–535. doi: 10.1111/j.1600-0765.1980.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Nair B. C., Mayberry W. R., Dziak R., Chen P. B., Levine M. J., Hausmann E. Biological effects of a purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983 Jan;18(1):40–49. doi: 10.1111/j.1600-0765.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Poirier T. P., Mishell R., Trummel C. L., Holt S. C. Biological and chemical comparison of butanol- and phenol-water extracted lipopolysaccharide from Capnocytophaga sputigena. J Periodontal Res. 1983 Sep;18(5):541–557. doi: 10.1111/j.1600-0765.1983.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Progulske A., Holt S. C. Isolation and characterization of the outer membrane and lipopolysaccharide from Eikenella corrodens. Infect Immun. 1984 Jan;43(1):166–177. doi: 10.1128/iai.43.1.166-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. R., Tashjian A. H., Jr, Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975 Nov;56(5):1181–1188. doi: 10.1172/JCI108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Stinson F. L., Parker R. B. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972 May;43(5):270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- Shaw D. H., Squires M. J., Ishiguro E. E., Trust T. J. The structure of the heptose-3-deoxy-D-mannooctulosonic-acid region in a mutant form of Aeromonas salmonicida lipopolysaccharide. Eur J Biochem. 1986 Dec 1;161(2):309–313. doi: 10.1111/j.1432-1033.1986.tb10448.x. [DOI] [PubMed] [Google Scholar]
- Smith A. R., Zamze S. E., Munro S. M., Carter K. J., Hignett R. C. Structure of the sidechain of lipopolysaccharide from Pseudomonas syringae pv. morsprunorum C28. Eur J Biochem. 1985 May 15;149(1):73–78. doi: 10.1111/j.1432-1033.1985.tb08895.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Dzink J. L., Ebersole J. L., Socransky S. S. Wolinella recta, campylobacter concisus, bacteroides gracilis, and Eikenella corrodens from periodontal lesions. J Periodontal Res. 1987 Jul;22(4):327–330. doi: 10.1111/j.1600-0765.1987.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Dowell V. R., Jr, Offenbacher S., Snyder W., Hersh T. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun. 1986 Sep;53(3):671–677. doi: 10.1128/iai.53.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walla M. D., Lau P. Y., Morgan S. L., Fox A., Brown A. Capillary gas chromatography-mass spectrometry of carbohydrate components of legionellae and other bacteria. J Chromatogr. 1984 Apr 24;288(2):399–413. doi: 10.1016/s0021-9673(01)93716-1. [DOI] [PubMed] [Google Scholar]
- Wightman P. D., Raetz C. R. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984 Aug 25;259(16):10048–10052. [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]